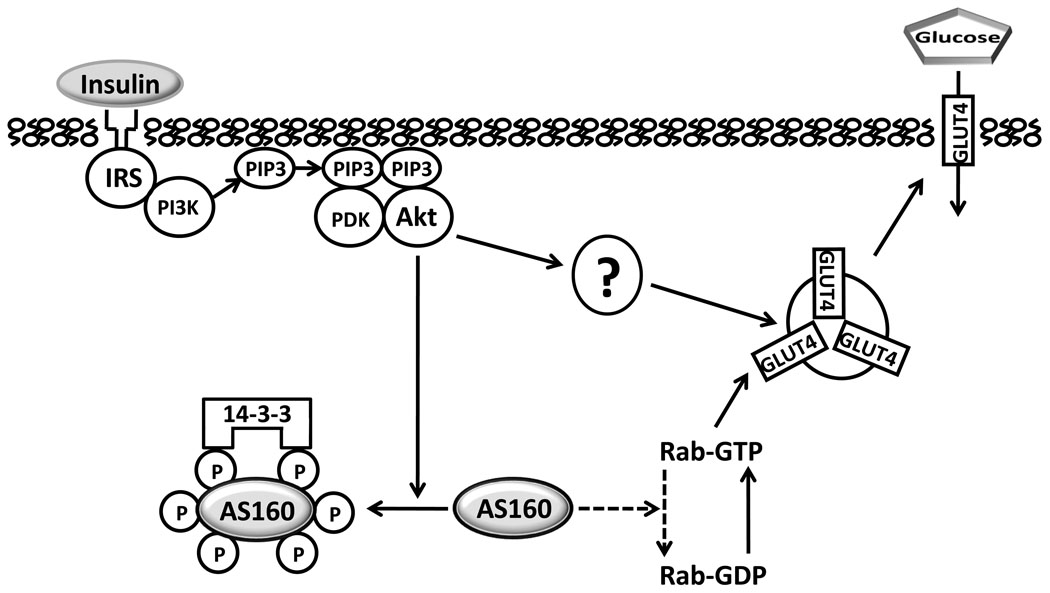
Figure 1.
Under basal conditions, the Rab GTPase activating domain of non-phosphorylated Akt Substrate of 160 kDa (AS160) catalyzes hydrolysis of Rab-bound guanosine triphosphate (GTP), producing Rab-bound guanosine diphosphate (GDP), which inhibits exocytosis of insulin responsive glucose transporter protein (GLUT4) vesicles. Insulin-stimulated, Akt-induced phosphorylation of AS160 releases this inhibition and results in more GTP-bound Rab, favoring GLUT4 translocation toward the cell surface. Proximal insulin signaling includes, insulin binding to its receptor, leading to tyrosine phosphorylation of insulin receptor substrates (IRS) which bind phosphatidylinositol-3 kinase (PI3K) and catalyze production of phosphatidylinositol 3,4,5-phosphate (PIP3) which binds phosphoinsitide-dependent kinase (PDK) and Akt, causing phosphorylation of Akt, which in turn, phosphorylates Akt Substrate of 160 kDa (AS160) on multiple Akt-phosphomotifs. Members of the 14-3-3 family of proteins bind to specific phosphomotifs of AS160, and this appears to promote AS160 release from membranes and/or to inhibit Rab GAP activity. There also appear to be unidentified Akt-dependent, but AS160 dependent mechanisms (indicated by “?”). Arrows with solid lines indicate a mechanism favoring increased GLUT4 translocation and glucose transport. Dashed lines indicate a mechanism favoring retention of intracellular GLUT4 and lower glucose transport.