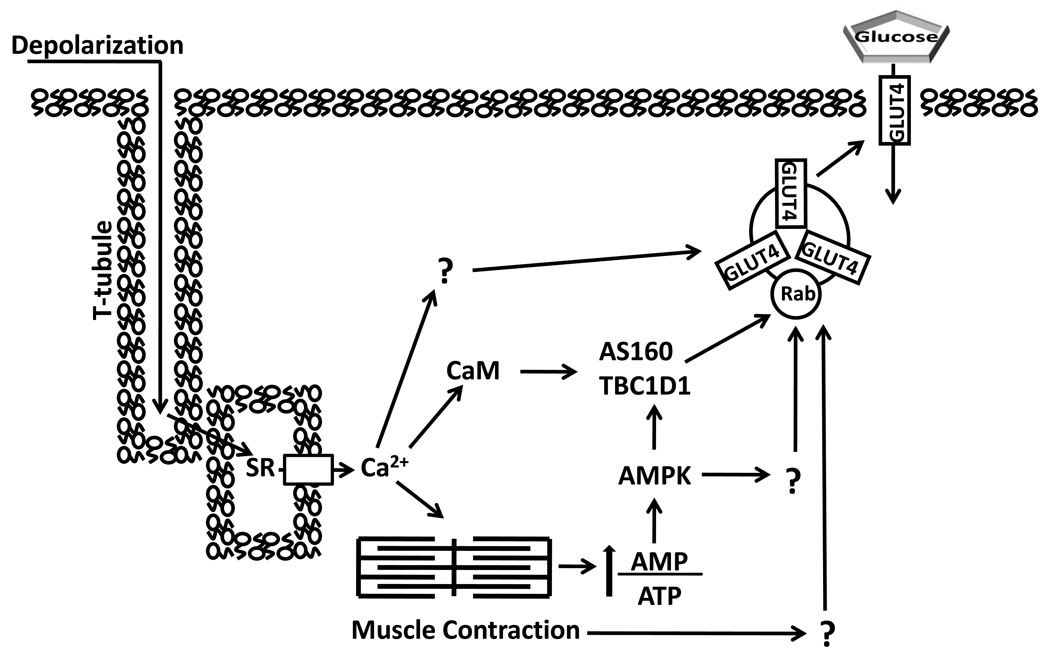
Figure 3.
Contraction stimulation of skeletal muscle glucose transport involves multiple inputs that lead to increased cell surface insulin responsive glucose transporter protein (GLUT4). T-tubule depolarization causes calcium (Ca2+) release from the sarcoplasmic reticulum (SR) which triggers actin and myosin interaction. The energy demand of contraction increases the ratio of adenosine monophosphate (AMP)/ adenosine triphosphate (ATP) which stimulates AMP-associated protein kinase (AMPK). AMPK can phosphorylate both AS160 and TBC1D1, as well as other unknown substrates, which potentially contribute to increased glucose transport. Both Akt substrate of 160 kDa (AS160) and TBC1D1 have a calmodulin (CaM) binding domain (CBD). The CBD of AS160 has been implicated in contraction-stimulated glucose transport, but the role of TBC1D1’s CBD is unknown. Increased glucose transport with contraction may also involve other Ca2+-dependent and Ca2+-independent processes (indicated by “?”).TBC1D indicates tre-2/USP6, BUB2, cdc16 domain.