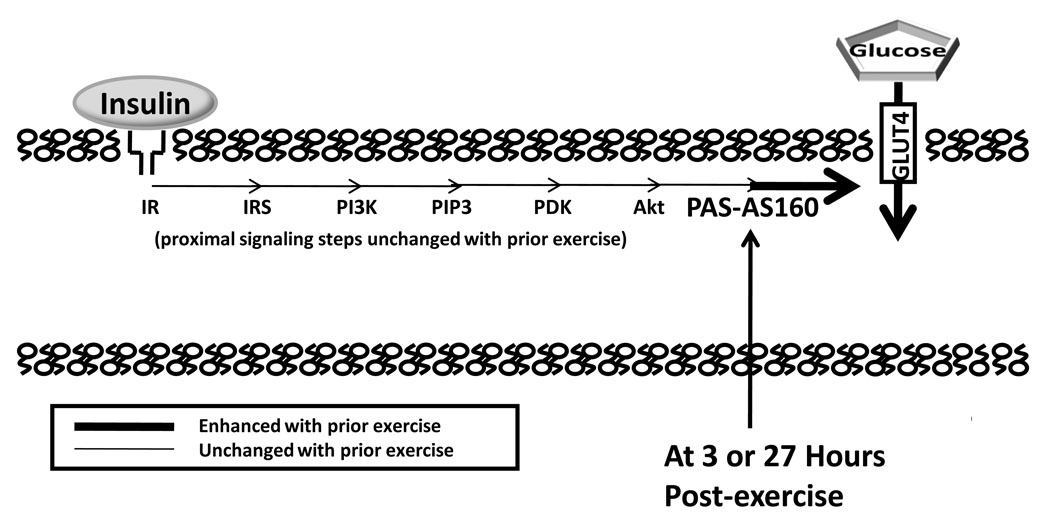
Figure 4.
Increased insulin-stimulated glucose transport, as the result of greater insulin responsive glucose transporter protein (GLUT4) translocation, can occur a few or many hours after acute exercise. A number of proximal insulin signaling steps (insulin receptor, IR, binding; tyrosine phosphorylation of IR and insulin receptor substrates, IRS; IRS-associated phosphatidylinositol-3 kinase, PI3K, activity; and Akt serine phosphorylation) are not enhanced a few hours after acute exercise. At either 3 or 27 hours after acute exercise, Akt Substrate of 160 kDa (AS160) phosphorylation on sites recognized by anti-PAS (PAS-AS160) was found to be increased in rat skeletal muscle in the absence of insulin (4, 12). We hypothesize that the persistent increase in AS160 phosphorylation plays a role in the increased insulin-stimulated glucose transport after acute exercise. PIP3 indicates phosphatidylinositol 3,4,5-phosphate; PDK, phosphoinsitide-dependent kinase; PAS, phospho-(Ser/Thr) Akt substrate.