Abstract
Objective: In this paper we compared the two methods of cell sorting (magnetic cell sorting and flow cytometry sorting) for the isolation and function analysis of mouse CD4+ CD25+ regulatory T (Treg) cells, in order to inform further studies in Treg cell function. Methods: We separately used magnetic cell sorting and flow cytometry sorting to identify CD4+ CD25+ Treg cells. After magnetic cell separation, we further used flow cytometry to analyze the purity of CD4+ CD25+ Treg cells, trypan blue staining to detect cell viability, and propidium iodide (PI) staining to assess the cell viability. We detected the immune inhibition of CD4+ CD25+ Treg cells in the in vitro proliferation experiments. Results: The results showed that compared to flow cytometry sorting, magnetic cell sorting took more time and effort, but fewer live cells were obtained than with flow cytometry sorting. The CD4+ CD25+ Treg cells, however, obtained with both methods have similar immunosuppressive capacities. Conclusion: The result suggests that both methods can be used in isolating CD4+ CD25+ Treg cells, and one can select the best method according to specific needs and availability of the methodologies.
Keywords: CD4+ CD25+ Treg cells, Flow cytometry sorting, Magnetic cell sorting
INTRODUCTION
CD4+ CD25+ regulatory T (Treg) cells are classified into mature Treg cells and induction Treg cells, and their functions in autoimmune diseases, transplantation tolerance, tumor immunity, infectious diseases, sepsis, and many other aspects have attracted more and more attention (Campbell and Ziegler, 2007; Carrigan et al., 2009; Ono et al., 2007; Xia et al., 2009). Treg cells have two characteristics: immune inability and immune inhibition. Many features of Treg cells’ immune response are different from those of other T cell subsets (Campbell and Ziegler, 2007; Ono et al., 2007). Mice are the most commonly used animals in the study of transplantation tolerance, autoimmune diseases, cancers, infections, and other diseases. Obtaining a higher yield of CD4+ CD25+ Treg cells with high cell activity in mice is a growing concern in immunology. In this article we compare the CD4+ CD25+ Treg cells obtained using magnetic cell sorting (MACS) versus flow cytometry sorting (FCS), and analyze their immunomodulatory effects, respectively.
MATERIALS AND METHODS
Main reagents
Anti-mCD4-fluorescein isothiocyanate (FITC) (IgG2) and anti-mCD25-phycoerythrin (PE) (IgG1) were purchased from the US Company, BD Pharmingen. Goat anti-mouse IgG beads, anti-mPE magnetic beads, Midi MACS sorting device, LD, and MS separation columns were purchased from the German Company, Mihenyi Biotech. Propidium iodide (PI) was purchased from Sigma (USA), and trypan blue moss was purchased from Beijing Biological Reagent Company (China). We used a phosphate buffer solution (PBS) with pH 7.2, and autoclave sterilization filter hemolysin saved at 4 °C.
Experimental animals
Specific pathogen-free (SPF) level BALB/c (H-2Dd) and C57BL/6 (H-2Db) mice were purchased from the Laboratory Animal Center of the Fourth Military Medical University, Xi’an, China. All animals were housed in plastic cages with controlled light/dark cycles and provided with food and water ad libitum. Sentinel animals in the same room were consistently surveyed serologically and were negative for infection agents. All experiments were performed according to approved animal care protocols.
Preparation of spleen lymphocytes
The mouse spleen separation was carried out in sterile conditions. We put the spleen in 10 ml PBS solution, then placed it in 200 mesh aseptic milling steel line, repeated milling, filtered twice, and then spent 10 min to centrifuge the spleen at 1500 r/min under 4 °C. Then we washed it by PBS twice, added the mouse lymphocyte separating medium, and centrifuged at 1500 r/min for 20 min to absorb the mononuclear cell layer. Finally, we washed it by PBS solution twice (adjusted cell concentration to 1×108 ml−1).
Isolation of CD4+ CD25+ Treg cells with magnetic cell sorting
We added the mice spleen single cell suspension into the “antibody cocktail” (10 μg for each antibody). After 20 min incubation under 4 °C, and 1500 r/min centrifugation for 10 min, we discarded the supernatant, re-suspended it by adding PBS solution, put an LD separation column in Midi MACS sorting device, washed the LD separation column twice with 1.0 ml of PBS solution, and then added the cell suspension to the separation column to obtain the cells flowing from the sorting column. We washed with PBS once, added 10 μg anti-mCD25-PE antibody to it, and incubated it under 4 °C for 10 min. Then we centrifuged the suspension at 1500 r/min for 10 min, discarded the supernatant, and then washed it again. We added 20 μl anti-PE magnetic beads. After incubation for 15 min under 4 °C, and centrifugation at 1500 r/min, we discarded the supernatant, re-suspended, placed it on an MS column in the Midi MACS sorting device, and then washed the separation column twice with 0.5 ml buffer solution. Next, we added the cell suspension in a separation column to obtain the CD4+ CD25− Treg cells flowing from the sorting column. We removed MS separation column from the magnetic field, and washed the double positive cells, and then the CD4+ CD25+ Treg cells were slowly out of the MS separation column (Loser et al., 2006). Finally, we took samples and detected the purity of double positive cells with flow cytometry.
Isolation of CD4+ CD25+ Treg cells with flow cytometry sorting
We added anti-mCD4-FITC and anti-mCD25-PE antibodies to the mice single cell suspension, incubated it under 4 °C for 10 min, centrifuged it at 1500 r/min for 10 min, discarded the supernatant, and then washed it again, adjusting the cell concentration to 1×107 ml−1. We sorted CD4+ CD25+ Treg cells with flow cytometry, and collected CD4+ CD25+ Treg cells and CD4+ CD25− T cells separately in the two flow tubes (Lu et al., 2008; Milner et al., 2007).
Cell survival evaluation by trypan blue staining
We calculated the mortality rate of CD4+ CD25+ Treg cells using conventional trypan blue staining, under an optical microscope.
Cell survival evaluation by PI staining
The CD4+ CD25+ Treg cells obtained from sorting were stained with PI. Mortality rate changes were detected with flow cytometry.
Immune regulation function analysis of in vitro CD4+ CD25+ Treg cells
CD4+ CD25+ Treg cells and CD4+ CD25− T cells of C57BL/6 mice received from sorting were then counted. We took spleen macrophages of BALB/c mouse, treated it with mitomycin C for 30 min under 37 °C, and then washed this twice with RPMI1640, counted and performed a back-up. We took BALB/c spleen macrophages as a means of stimulating cells (1×105 cells) and C57BL/6 CD4+ CD25− T cells (1×105 cells) as reaction cells. We also observed the changes in immune regulation effects of CD4+ CD25+ Treg cells obtained from both sorting methods (Golshayan et al., 2007).
Statistical analysis
The data were analyzed with CELL QUEST software. The values of standardized samples in each group were presented with mean±SD, SPSS 12.0 statistical software package was used for Student’s t-test analysis, and P<0.05 indicated a statistically significant difference.
RESULTS
Purity detection of the CD4+ CD25+ Treg cells sorted with the two different methods
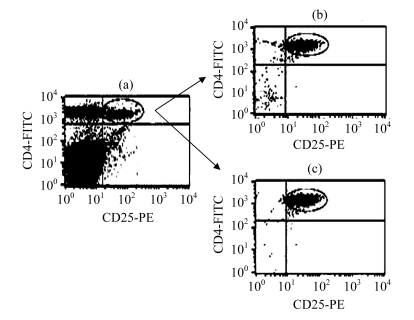
Using the MACS method, we obtained 5×105 cells in 6 to 8 h of sorting, with a purity of (91.2±6.2)%, detected by flow cytometry staining. But sorting with FCS for 3 to 4 h, we obtained the same number of cells, with a purity up to (99.0±0.5)% detected by cell staining. Thus, FCS provided a higher purity than the MACS method, with a shorter sorting time (Fig.1).
Fig.1.
Purity identification of the CD4+ CD25+ Treg cells by the two sorting methods
(a) The content of CD4+ CD25+ Treg cells in spleen lymphocytes; (b) The purity of CD4+ CD25+ Treg cells by magnetic cell sorting; (c) The purity of CD4+ CD25+ Treg cells by flow cytometry sorting
Activity detection of the CD4+ CD25+ Treg cells sorted with two methods
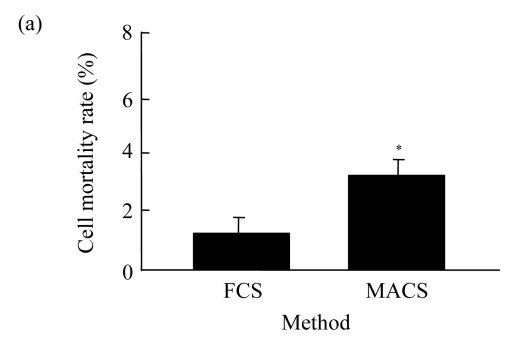
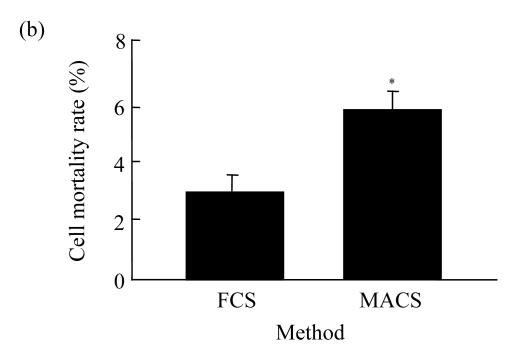
The mortality rate of CD4+ CD25+ Treg cells gained from FCS method was significantly lower (P<0.05) than that from MACS method as judged by trypan blue staining (Fig.2a). Meanwhile, using PI staining, the mortality rate of cells obtained from the FCS method was also significantly lower (P<0.05) than that from MACS method (Fig.2b).
Fig.2.
Death detection of CD4+ CD25+ Treg cells with the two methods
(a) Trypan blue staining; (b) PI staining (* P<0.05)
Analysis of the immune regulation function of the CD4+ CD25+ Treg cells obtained by sorting
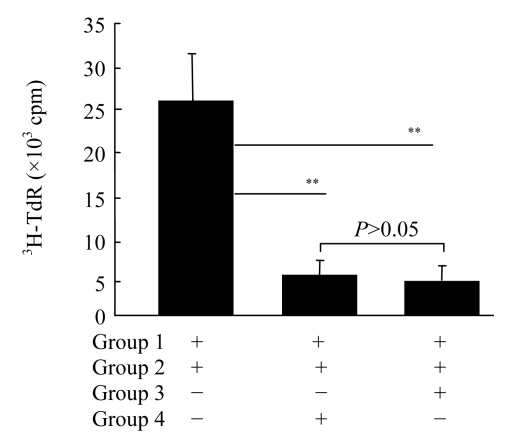
We sorted the spleen macrophages of BALB/c mouse, and used them as a means of stimulating cells, and CD4+ CD25− T cells of C57BL/6 mouse spleen as reaction cells. We established the reaction system of the same allogeneic mixed lymphocyte culture, in order that the difference in immune modulation effect of CD4+ CD25+ Treg cells obtained through both methods could be observed. The results showed that CD4+ CD25+ Treg cells obtained from either FCS and MACS could inhibit the proliferative response of the same allogeneic T cells in BALB/c mouse, and the inhibitory effect showed no significant difference (P>0.05) (Fig.3).
Fig.3.
Immunosuppressive function detection of sorting cells with both two methods
3H-TdR: [3H] thymidine; cpm: counts per minute. Group 1: BALB/c spleen macrophage stimulates; Group 2: C57BL/6 CD4+ CD25− T cells; Group 3: (FCS) C57BL/6 CD4+ CD25+ Treg cells; Group 4: (MACS) C57BL/6 CD4+ CD25+ Treg cells. ** P<0.01
Comparison of the relevant indexes of the two sorting methods
The difference between the relevant indexes of CD4+ CD25+ Treg cells sorted with both methods was analyzed in terms of the overall results of the experiment (Table 1).
Table 1.
Comparison of MACS and FCS in sorting CD4+ CD25+ Treg cells
| Method | Sorting purity (%) | Sorting time (h) | Sorting technical requirements | Cell sorting activity | Cell sorting function | Cell sorting equipment | Application scope |
| MACS | 91.2±6.2 | 6~8 | A higher level sorting requirement | High | Normal | Reagent kits | Sort a small number of Treg cells |
| FCS | 99.0±0.5 | 3~4 | Basic sorting technology | Higher | Normal | FCS equipment | Sort a large number of Treg cells |
DISCUSSION
CD4+ CD25+ Treg cells play an important regulatory role in immune regulation, the maintenance of peripheral immune tolerance, and the prevention of autoimmune diseases. Their role in immune regulation has become a much discussed topic in immunology (Steger et al., 2006; Wang et al., 2006). Therefore, to obtain more pure and highly active CD4+ CD25+ Treg cells is an essential goal of research in this area. Our results show that the sorting time of the MACS method is almost twice that of FCS method, but its purity is lower than that of the FCS method. The standard deviation of CD4+ CD25+ Treg cell purity gained from MACS method is larger than that of FCS method. This may be caused by operator variability since the operator of the MACS method uses more advanced technology. Moreover, different operators, due to different operation techniques, may have a significant effect on the purity of CD4+ CD25+ Treg cells. Furthermore, the same operator in different operations may affect the determination of purity of sorting (unpublished data). However, CD4+ CD25+ Treg cells sorted by the FCS method are less affected by the operator, and the sorting purity is higher and more stable.
We detected the activity of CD4+ CD25+ Treg cells after they were sorted with both methods. The results showed that the mortality rate of CD4+ CD25+ Treg cells obtained from the FCS method was significantly lower than that of MACS method. This suggests that FCS method can shorten the operation time and avoid operator-based factors that significantly increase the mortality rate. This is in contrast to the higher mortality rate of the MCS method.
The immune regulatory function of CD4+ CD25+ Treg cells sorting with both methods showed that CD4+ CD25+ Treg cells sorted by both methods could both inhibit the same allogeneic mixed lymphocyte proliferative response. The CD4+ CD25+ Treg cells sorted by the two methods had no significant difference in cellular immunosuppression function. This suggests that CD4+ CD25+ Treg cells sorted by either method have a similar immune regulation function, and both can be used for experimental study of the immune regulation function.
Reagents used by MACS method to sort CD4+ CD25+ Treg cells were mainly purchased from foreign biological reagent companies. In order to sort regulatory T cells by FCS, it is necessary to purchase immunofluorescent staining antibodies and FCS equipments with the function of cell sorting. Comparatively, it appears that FCS is simpler, more flexible and applicable to situations with smaller numbers of CD4+ CD25+ Treg cells. As well, FCS can be used when there are larger numbers of CD4+ CD25+ Treg cells, achieving a high purity. In short, although both methods have advantages and disadvantages, the CD4+ CD25+ Treg cells are sorted with the either method can reflect the functional characteristics of regulatory T cells well and objectively; this is less problematic with FCS.
Acknowledgments
We appreciate Master Yan ZHENG and Ni-ya AN (Wuhan Fenghe Medicine Science and Technology Development Co. Ltd., Wuhan, China) for their critical review of the manuscript, and Ms. Xiao-li HE and Mr. Qi GUO for their excellent laboratory management.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30872578 and 30753761), the Natural Science Foundation of Shanxi Province (No. SJ08C201), the Science and Technology Key Projects Foundation of Shanxi Province (No. 2008K13-04), and the Science and Technology Plan Projects Foundation of Xi’an (No. SF08006-2), China
References
- 1.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nature Reviews Immunology. 2007;7(4):305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 2.Carrigan SO, Yang YJ, Issekutz T, Forward N, Hoskin D, Johnston B, Lin TJ. Depletion of natural CD4(+)CD25(+) T regulatory cells with anti-CD25 antibody does not change the course of Pseudomonas aeruginosa-induced acute lung infection in mice. Immunobiology. 2009;214(3):211–222. doi: 10.1016/j.imbio.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Golshayan D, Jiang SP, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4(+)CD25(+) regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 4.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nature Medicine. 2006;12(12):1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 5.Lu YQ, Huang WD, Cai XJ, Gu LH, Mou HZ. Hypertonic saline resuscitation reduces apoptosis of intestinal mucosa in a rat model of hemorrhagic shock. Journal of Zhejiang University-SCIENCE B. 2008;9(11):879–884. doi: 10.1631/jzus.B0820116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446(7136):685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 8.Steger U, Kingsley CL, Karim M, Wood KJ. CD25(+)CD4(+) regulatory T cells develop in mice not only during spontaneous acceptance of liver allografts but also after acute allograft rejection. Transplantation. 2006;82(9):1202–1209. doi: 10.1097/01.tp.0000235913.58337.b4. [DOI] [PubMed] [Google Scholar]
- 9.Wang HJ, Zhao L, Sun ZY, Sun LG, Zhang BJ, Zhao Y. A potential side effect of cyclosporin A: inhibition of CD4(+)CD25(+) regulatory T cells in mice. Transplantation. 2006;82(11):1484–1492. doi: 10.1097/01.tp.0000246312.89689.17. [DOI] [PubMed] [Google Scholar]
- 10.Xia GL, Shah M, Luo XR. Prevention of allograft rejection by amplification of Foxp3+CD4+CD25+ regulatory T cells. Translational Research. 2009;153(2):60–70. doi: 10.1016/j.trsl.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]