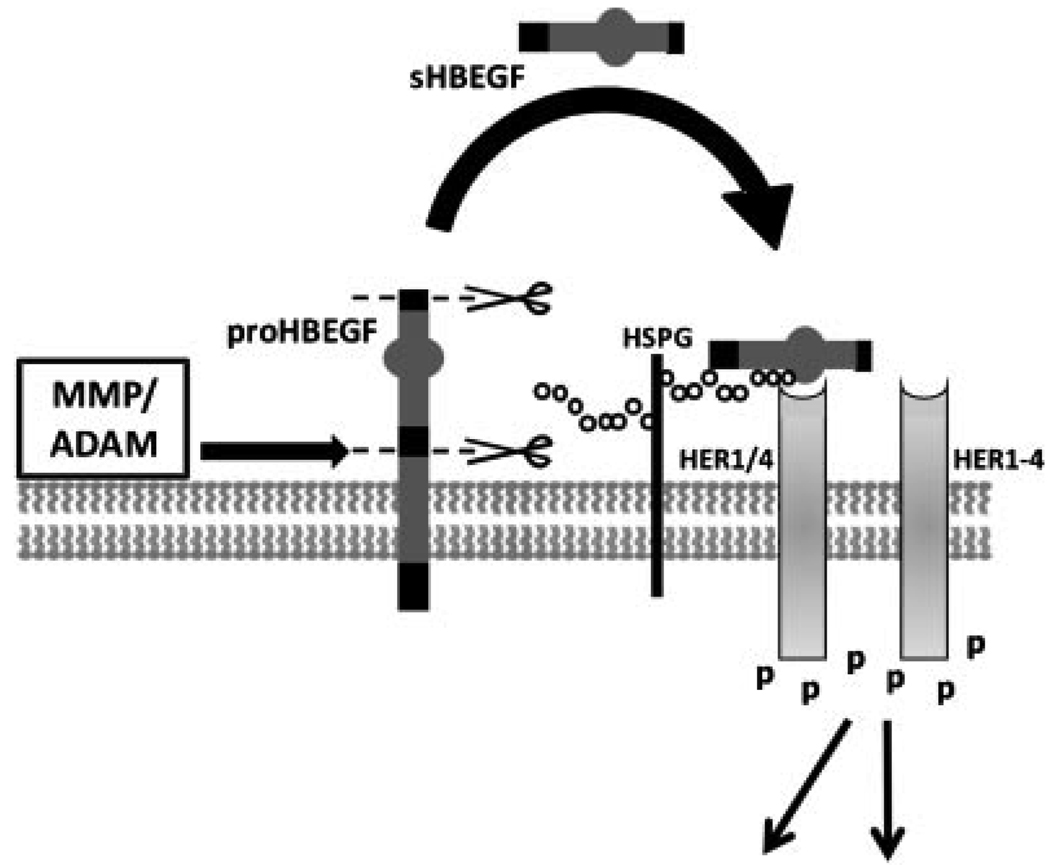
Figure 1.
HBEGF processing and signaling. In its transmembrane form, proHBEGF is proteolytically cleaved by metalloproteinases at the cell surface to remove (scissors) its pro-domain and secrete the extracellular domain (sHBEGF shedding). sHBEGF is then free to diffuse to nearby receptor tyrosine kinases where it can bind either HER1 (EGF receptor) or HER4. Receptor binding requires heparin sulfate proteoglycan (HSPG) as cofactor, and induces HER homo- or heterodimerization. Receptor autophosphorylation initiates down-stream intracellular signaling. Heparin sulfate proteoglycan (HSPG); human epidermal growth factor receptor family member (HER); matrix metalloproteinase (MMP); a disintegrin and metalloproteinase (ADAM).