Abstract
Atrial natriuretic peptide (ANP) is a hormone with diuretic, natriuretic, and vasodilatory properties. ANP blocks vascular endothelial growth factor (VEGF) production and signaling in vitro; however, its role in vascular leakage and angiogenesis is unknown. In vitro, retinal barrier permeability (transepithelial electrical resistance (TEER)) was measured in cultured retinal endothelial (HuREC) and retinal epithelial (ARPE-19) cells with VEGF (10 ng/ml), ANP (1 pM to 1 μmol/L), and/or isatin, an ANP receptor antagonist. In vivo, blood-retinal barrier (BRB) leakage was studied using the Evans Blue dye technique in rats treated with intravitreal injections of ANP, VEGF, or vehicle. Choroidal neovascularization was generated by laser injury, and 7 days later, lesion size and leakage was quantitated. ANP significantly reversed VEGF-induced BRB TEER reduction in both HuREC and ARPE-19 cells, modeling the inner and the outer BRB, respectively. Isatin, a specific ANP receptor antagonist, reversed ANP’s effect. ANP reduced the response of ARPE-19 cells to VEGF apically but not basolaterally, suggesting polarized expression of the ANP receptors in these cells. ANP’s TEER response was concentration but not time dependent. In vivo, ANP significantly reduced VEGF-induced BRB leakage and the size of laser-induced choroidal neovascularization lesions. In sum, ANP is an effective inhibitor of VEGF-induced vascular leakage and angiogenesis in vivo. These results may lead to new treatments for ocular diseases where VEGF plays a central role, such as age-related macular degeneration or diabetic retinopathy.
Currently, >1.75 million people suffer from age-related macular degeneration (AMD) in the United States, and this number is estimated to grow to 3 million by the year 2020.1 AMD occurs in two forms, wet and dry. In wet AMD, choroidal vessels pathologically grow through the retinal pigmented epithelial (RPE) cell layer into the subretinal space, a process known as choroidal neovascularization (CNV). As opposed to normal retinal vessels that exhibit a strong barrier function, known as the blood-retina barrier (BRB), the CNV vessels are leaky. CNV and the ensuing leakage damage the RPE and retinal cells, leading to permanent vision loss.2,3,4,5 Vascular endothelial growth factor (VEGF) is a key molecule responsible for the growth and subsequent leakiness of CNV.6
Currently, the leading treatment of AMD is based on local inhibition of VEGF, using monoclonal antibody fragments (Ranibizumab; Lucentis).7,8,9 However, recent evidence suggests that VEGF is required for normal retinal physiology, raising concerns about the long-term use of VEGF inhibitors.10
Natriuretic peptides (NPs) are cardiovascular cyclic peptide hormones with diuretic, natriuretic and vasodilatory properties.11 The NP family consists of three members, atrial NP (ANP), brain NP, and C-type NP. ANP is primarily produced in the cardiac atria and is used in the treatment of various disorders, including hypertension, renal insufficiency, and congestive heart failure.11,12 However, ANP is also expressed in the human retina, in the inner plexiform layer, and in the RPE.13
The action of NPs is mediated through two types of receptors: guanylate cyclase type A, which reacts with ANP and brain NP, and guanylate cyclase type B, which is C-type NP-specific.14,15 Binding of the NPs to these receptors results in cGMP production, which activates protein kinase G and subsequent target genes.11 Recently, ANP was found to be antiangiogenic in vitro, because it antagonizes both VEGF production and signaling.16 ANP inhibits the VEGF-induced activation of Erk, c-Jun N-terminal kinase, and p-38 in a concentration-dependent fashion, thus reducing endothelial tube formation and proliferation.16 However, the role of ANP in ocular diseases has not been investigated. This study explores the role of ANP in VEGF-mediated retinal vascular leakage and angiogenesis.
Materials and Methods
Animals
All animal experiments adhered to the Association of Research in Visual Sciences and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Experimental protocols were approved by the Animal Care Committee of the Massachusetts Eye & Ear Infirmary. Male Brown Norway rats (Charles River Laboratories, Wilmington, MA), weighing 200 to 250 g, and 4-month-old C57BL/6 mice were used in the experiments. Animals were sheltered in plastic cages in a temperature-controlled animal facility with a 12-hour light/dark cycle and were fed standard laboratory chow and water ad libitum.
Intravitreal Injections
Animals were anesthetized by an intramuscular injection of xylazine hydrochloride (6 mg/kg; Phoenix Pharmaceutical, St. Joseph, MO) and ketamine hydrochloride (40 mg/kg; Parke-Davis, Morris Plains, NJ). To perform the intravitreal injections, a 31-gauge needle (Hamilton) was used, with the insertion 1 mm posterior to the corneal limbus.17 VEGF, ANP, or both were injected into the vitreous. Insertion and injections were performed under an operating microscope to avoid contact of the needle with the lens or retina. Eyes that exhibited signs of lens or retinal injury were excluded.
Laser-Induced CNV
To induce CNV, C57BL/6 mice and Brown Norway rats were anesthetized, and pupils were dilated with 5% phenylephrine and 0.8% tropicamide.18 Using a 532-nm laser (Oculight GLx; Iridex, Mountain View, CA) , a slit-lamp delivery system, and a cover glass as a contact lens, four spots were placed in each eye. The parameters were 100 mW, 50 μm, and 100 ms in mice and 150 mW, 100 μm, 100 ms in rats. The lesions were located at the 3, 6, 9, and 12 o’clock meridians centered on the optic nerve head and located ∼2 to 3 disk diameters from the optic nerve head. Development of a bubble under laser confirmed the rupture of the Bruch’s membrane. Eyes showing hemorrhage were excluded from experiments.
Evaluation of CNV and Leakage
Seven days after laser injury, the size of the CNV lesions was measured in choroidal flat mounts.18 Briefly, animals were anesthetized and perfused through the left ventricle with PBS, followed by 5 ml of fluorescein-labeled dextran (5 mg/ml, fluorescein isothiocyanate-dextran; Sigma Aldrich) in 1% gelatin. The eyes were enucleated and fixed in 4% paraformaldehyde for 3 hours. Anterior segment and retina were removed from the eyecup. The remaining RPE-choroid-sclera complex was flat mounted after four relaxing radial incisions using Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA) and coverslips. Micrographs of the choroid complex were taken using a Leica Microscope (Leica, Wetzlar, Germany). The magnitude of the CNV lesions was determined by measuring the hyperfluorescent area using Openlab Software (Improvision, Boston, MA).
Fluorescein Angiography
Seven days after laser injury, vascular leakage from the CNV lesions was assessed using fluorescein angiography (FA), as described previously.18 Briefly, FA was performed in anesthetized animals from ANP-treated or control groups, using a digital fundus camera (TRC 50 IA; Topcon, Paramus, NJ). Fluorescein injections were performed i.p. (0.2 ml of 2% fluorescein solution; Akorn, Decatur, IL). FA images were evaluated by two masked retina specialists, as described previously.18 Briefly, the grading criteria were as follows: grade 0 lesions had no hyperfluorescence. Grade I lesions exhibited hyperfluorescence without leakage. Grade IIA lesions exhibited hyperfluorescence in the early or midtransit images and late leakage. The grade IIB lesions, hyperfluorescence that increased in intensity and in size during the transit phase of the angiogram, were defined as clinically significant.18
BRB Breakdown Measurement with the Evans Blue Technique
To quantify retinal vascular permeability, Evans blue (EB) dye (Sigma-Aldrich) (30 mg/ml in saline; Sigma-Aldrich) was injected through the tail vein of anesthetized rats over 10 seconds at a dosage of 45 mg/kg.19 Blood samples were obtained from the left ventricle, just before perfusion to obtain the time-averaged EB plasma concentration. To separate the plasma from the cellular components, blood samples were centrifuged at 12,000 rpm for 15 minutes. The plasma samples were diluted to 1/10,000th of their initial concentration in formamide (Sigma-Aldrich). The absorbance was measured with a spectrophotometer at 620 and 740 nm. After the dye had circulated for 2 hours, the chest cavity was opened, and the rats were perfused through the left ventricle with paraformaldehyde 1% in citrate buffer (0.05 M, pH 3.5) at a constant pressure of ∼120 mmHg. The retinas were then carefully dissected under an operating microscope. After measurement of the retinal weight, EB was extracted by incubating each retina in 180 μl of formamide for 18 hours at 70°C. The extract was ultracentrifuged for 60 minutes at 14,000 rpm and 25°C. Sixty microliters of the supernatant was used for spectrophotometry. The background-subtracted absorbance was determined by measuring each sample at 620 nm (the absorbance maximum for EB in formamide) and 740 nm (the absorbance minimum). BRB breakdown was calculated as previously described and values expressed as plasma (μl) × retinal weight (g)−1 × time (h)−1.20,21
Cell Culture
HuRECs were grown in endothelial cell growth medium 2 (C-22011; PromoCell), and ARPE-19 (American Type Culture Collections) were cultured according to vendor instructions and are detailed elsewhere.22 Barrier function of confluent monolayer cultures was assessed by transepithelial electrical resistance (TEER) measurements. The TEER was monitored with an epithelial volt-ohmmeter (WPI, Sarasota, FL) equipped with an STX2 electrode (WPI). Resistance values for individual wells were determined from at least four independent measurements and corrected for the inherent resistance of the membrane inserts. For each condition, three or more independent experiments were performed. Only confluent monolayer cultures with stable TEER values were used. Confluency was reached within 3 weeks after plating.22
Cell Treatments
VEGF (human VEGF-A165; Sigma-Aldrich) was administered to either the apical or basal sides of the membrane inserts with confluent monolayers (10 ng/ml) in the absence or presence of ANP (human, 1 to 28; Peptide International, Louisville, KY) (1 μmol/L). Baseline TEER was measured at −60 minutes and immediately before agonist administration (t = 0). After this treatment, resistance was measured at several time points and up to 4 days after treatment to obtain time course of the barrier function. ANP was used in 1 pM to1 μmol/L concentration range. To study the actions of the NP receptors, some cultures were pretreated 60 minutes before the above treatments with isatin (100 μmol/L; Sigma-Aldrich). Concentration curves were analyzed using Prism 4.02 software (GraphPad Software, San Diego, CA).
Statistical Analysis
Values are expressed as the mean ± SE and compared by the Student’s t-test. The results of the FA grading were statistically compared using the χ2 test. Values of P < 0.05 were considered statistically significant.
Results
ANP Reduces VEGF-Induced Retinal Vascular Leakage in Vitro
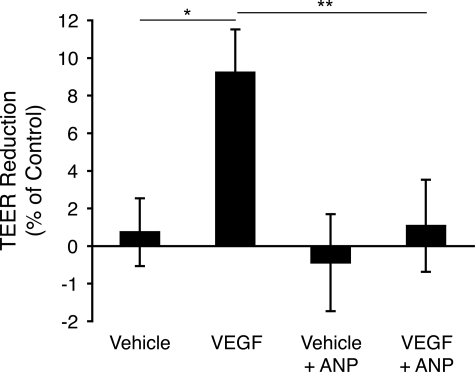
To investigate the role of ANP in the regulation of barrier function, TEER measurements were performed in human retinal vascular endothelial cells. In the eye, these cells are a key component of the inner BRB. They were treated with ANP (1 μmol/L) or vehicle in the presence or absence of VEGF (100 ng/ml), and TEER was measured 30 minutes after ANP administration. VEGF significantly reduced TEER in retinal vascular endothelial cell cultures, when compared with controls (10.1 ± 0.8% versus 4.3 ± 3.2%, n = 6, P = 0.02). Cotreatment of the cells with ANP effectively blocked this reduction, resulting in baseline TEER values (2.4 ± 2.8%, n = 6, P = 0.01) (Figure 1).
Figure 1.
ANP blocks VEGF-induced reduction of barrier function in retinal endothelial cells. To assess the effect of ANP on barrier properties, retinal endothelial cells in the confluent state were treated with VEGF (100 ng/ml) and/or ANP (1 μmol/L). Subsequently, TEER values were measured. Data represent comparisons of the leakage between VEGF and ANP treated eyes ±SEM, n = 6; *P = 0.015, **P = 0.03.
ANP Reduces VEGF-Induced Vascular Leakage in the Outer BRB
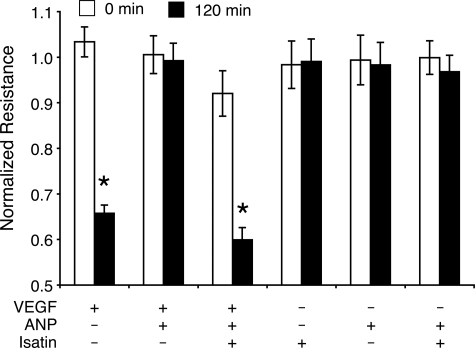
Next, the effect of ANP on RPE barrier function, which in the eye constitutes the outer BRB, was investigated. ARPE-19 cells were grown to monolayer cultures until they stabilized, and their TEER levels were assessed. VEGF (10 ng/ml) induced a reduction in the resistance of the RPE cell monolayer, similar to results previously obtained in these cells (the TEER values dropped by 34.3 ± 3%, 2 hours after addition of VEGF).22 In the presence of ANP (1 μmol/L), TEER levels remained at baseline values, indicating that coadministration of ANP effectively reverses VEGF-induced TEER reduction in ARPE-19 cells. To test the specificity of the ANP response, isatin, an NP receptor antagonist, was administered 60 minutes before ANP and VEGF. Pretreatment with isatin (100 μmol/L) completely reversed the inhibitory effects of ANP with respect to the VEGF-induced TEER reduction (measured at 120 minutes after agonist administration). In control experiments, isatin alone did not significantly affect TEER levels (Figure 2).
Figure 2.
ANP inhibits the effect of VEGF on RPE barrier function. ARPE-19 cells were grown to confluence, and TEER values were obtained in the presence or absence of VEGF (10 ng/ml) and ANP (1 μmol/L). ANP blocked VEGF-induced TEER drop. Treatment with isatin (100 μmol/L) completely inhibits ANP’s effect on VEGF-induced TEER reduction; *P < 0.05.
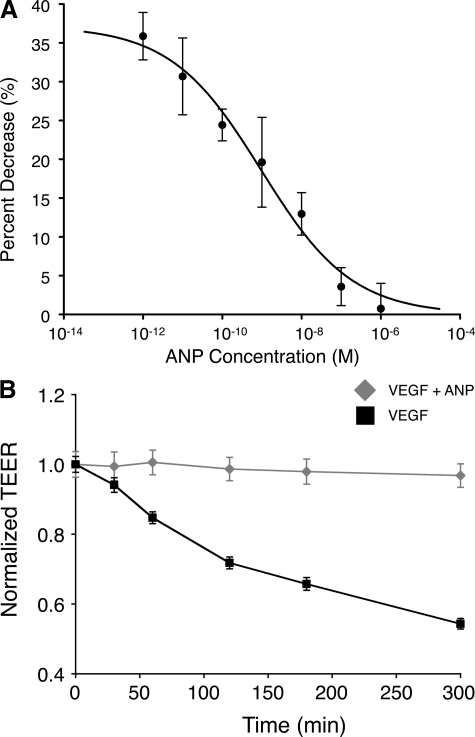
Time Course of ANP’s Effect on VEGF-Induced Leakage
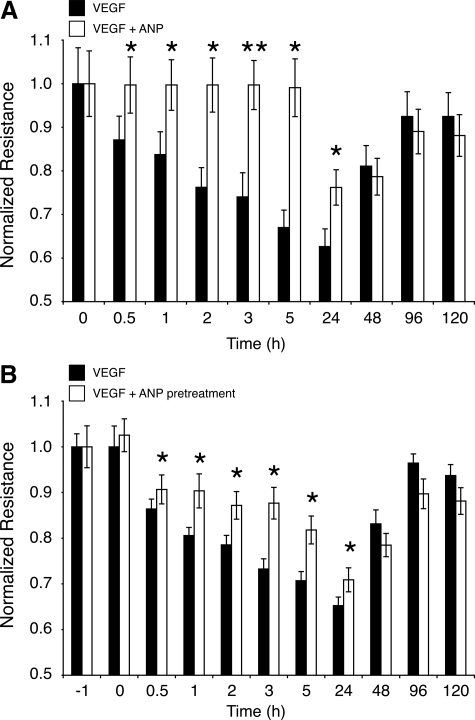
To investigate the duration of ANP’s impact on reversing VEGF-induced TEER drop, ARPE-19 cells were cotreated with VEGF (10 ng/ml) plus ANP (1 μmol/L) or VEGF alone. In VEGF-treated RPE cells, a significant TEER reduction was measurable by 0.5 hours (P < 0.05) that continued until 24 hours (P = 0.05) posttreatment. In comparison, in VEGF and ANP cotreated RPE cells, TEER did not significantly change by 5 hours, and it was by 24 hours still significantly higher than in VEGF-treated cells. After 24 hours, in both groups TEER started to increase and eventually reached normal levels (Figure 3A).
Figure 3.
Time course of ANP’s effect on VEGF-induced leakage. ARPE-19 cells were grown to confluence, and TEER values were measured after ANP incubation or preincubation treatments. A: Cells were incubated with VEGF (10 ng/ml) or VEGF + ANP (1 μmol/L). Data represent mean ± SEM, n = 3 in each group; *P < 0.05. B: Cells were preincubated with ANP, and subsequently, the medium was removed and the cells were then incubated with VEGF-containing medium. Data represent mean ± SEM, n = 3 in each group; *P < 0.05.
To investigate, whether ANP would have to be present in the extracellular medium to exert its effect, RPE cells were pretreated with ANP (1 μmol/L) for 1 hour; subsequently, the ANP-containing medium was removed, and the cells were incubated with VEGF (10 ng/ml). ANP-pretreated cells showed significantly less VEGF-induced compared with the VEGF-treated controls, suggesting a lasting effect of ANP on RPE (Figure 3B).
Polarized Response of RPE Barrier Function to ANP
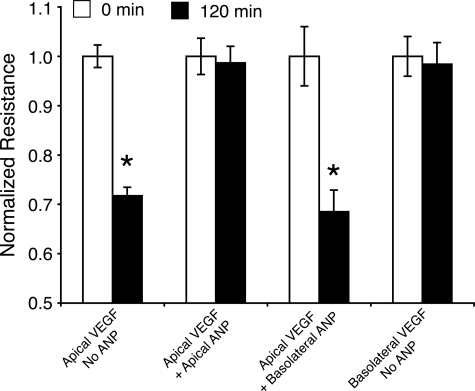
The ARPE-19 cell response to VEGF occurs only with apical administration.22 In line with the previous experiments, a reduction in the TEER was only observed with apical VEGF treatment. Two hours after apical VEGF (10 ng/ml) administration, TEER was reduced to 71.8 ± 1.7% of the initial levels. In contrast, basolateral VEGF treatment did not cause a TEER drop. To investigate the polarity of the ANP response, ARPE-19 cells were coadministered with apical VEGF and ANP from either apical or the basolateral side. Apical (but not basolateral) addition of ANP (1 μmol/L) blocked the apical VEGF response (Figure 4).
Figure 4.
Polarized response of the outer BRB to ANP and VEGF. VEGF (10 ng/ml) and/or ANP (1 μmol/L) were given to the monolayer cell culture, and 120 minutes later, TEER values were obtained. Apical administration of VEGF significantly reduced TEER. ANP blocked VEGF-induced TEER reduction when given apically but not basolaterally; *P < 0.05.
Concentration- but Not Time-Dependent ANP Response in RPE Cells
To characterize the role of ANP in VEGF-induced TEER reduction and to explore the signaling mechanisms involved in the process, we investigated the effect of ANP at different concentrations. ARPE-19 were treated with different ANP concentrations (1 pM to 1 μmol/L) in the presence of 10 ng/ml VEGF, and TEER measurements were obtained 2 hours after ANP administration to the cultures. The percent decrease in TEER was inversely proportional to the ANP concentration and followed a sigmoidal dose-response curve. The half maximal inhibitory concentration (IC50) was 1.02 nmol/L (logIC50 = −8.99 ± 0.4), and the Hill slope was −0.38. The fact that the Hill slope is far from −1 suggests that the reaction of ANP does not follow the law of mass action with a single site and that the ANP response may be mediated through multiple signaling mechanisms (Figure 5A).
Figure 5.
ANP’s effect is concentration but not time dependent. A: ARPE-19 cells were treated with different ANP concentrations (1 pM to 1 μmol/L) in the presence of VEGF (10 ng/ml). B: TEER measurements for up to 5 hours after RPE cells were treated with VEGF (10 ng/ml) in the presence or absence of ANP (1 μmol/L).
Kinetics studies were performed to determine the extent of the observed ANP inhibitory effects. In the presence of VEGF (10 ng/ml), normalized TEER declined as a function of time. Five hours after VEGF (10 ng/ml) administration, TEER values dropped to 54.3 ± 1.5% of the starting levels (at t = 0). However, this time-dependent decline in resistance was inhibited for the entire time period by a single treatment with ANP (1 μmol/L at t = 0) (Figure 5B).
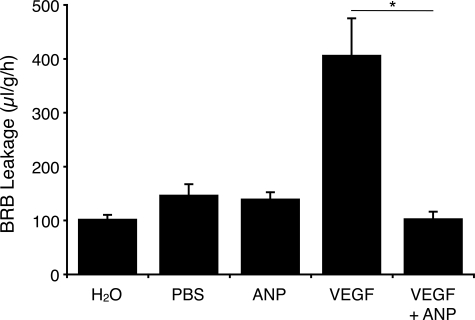
ANP Antagonizes VEGF-Induced Retinal Vascular Leakage
To study the effect of ANP on VEGF-induced BRB breakdown, rats were treated with intravitreous injections VEGF164 with or without ANP. Twenty-four hours after injections, retinal vascular permeability was quantified by the EB technique.
Intravitreous injection of VEGF (5 μl, 25 ng/μl) induced a significant, three-fold, increase in retinal vascular leakage compared with vehicle (PBS)-injected eyes (406.6 ± 68.7 versus 108.5 ± 7.7 ml/g/h, n = 9, P < 0.001) (Figure 6). To asses whether ANP reduces VEGF-induced BRB breakdown, rats were coadministered intravitreal ANP (5 μl, 10−4 M). The VEGF-induced BRB leakage was significantly suppressed by ANP coadministration (406.6 ± 68.7 versus 103.6 ± 13.1 ml/g/h, n = 9, P < 0.001) (Figure 6). Intravitreal injection of ANP alone did not affect BRB leakage, 24 hours after injection, compared with vehicle-treated controls (139.4 ± 12.5 versus 108.5 ± 7.7 μl/g/h, n = 9, P = 0.15) (Figure 6).
Figure 6.
ANP reduces BRB breakdown in vivo. To evaluate whether ANP blocks VEGF-induced retinal vascular permeability in vivo, 5 μl of ANP (25 ng/μl) was injected concomitantly with VEGF (5 μl, 25 ng/μl) intravitreally in one eye and the same volume of vehicle (5 μl of PBS) in the contra lateral eye. In other animals, 5 μl of VEGF (25 ng/μl) was injected intravitreally in one eye and the same volume of vehicle in the other eye. BRB breakdown was evaluated with the EB technique 24 hours after intravitreal injections. Bars represent mean ± SEM; n = 9, *P < 0.01.
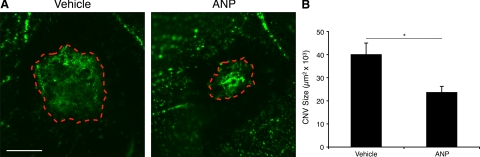
The Role of ANP in CNV Formation
To investigate the role of ANP in CNV formation, the fundus of C57BL/6 mice was photocoagulated with or without intravitreal injection of ANP (50 ng). Seven days after laser injury, the CNV areas were quantified in flat-mounted RPE-choroid tissues. ANP-treated animals showed significant decrease in CNV size, when compared with vehicle-treated animals (39,991 ± 5,411 versus 23,606 ± 2,847 μm2 n = 7, P = 0.013) (Figure 7, A and B).
Figure 7.
Role of ANP in CNV formation. To asses whether ANP inhibits CNV formation, fundi of the mice eyes were photocoagulated and treated with intravitreal injections of ANP or vehicle as control. A: Representative micrographs of CNV lesions in the choroidal flat mounts from a control and ANP-treated animal. Bar, 100 μm. B: Quantitative analysis of CNV size. ANP treated, n = 18; vehicle control, n = 20. Bars show the average of CNV size in each group. Data represent mean ± SEM; *P = 0.013.
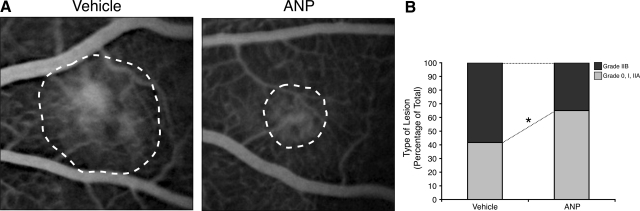
To quantify the leakage in the CNV lesions, we performed FA, 7 days after laser injury (Figure 8A). The incidence of clinically significant CNV lesions, grade IIB, was significantly lower in ANP-treated animals (35%, n = 20) compared with vehicle-treated controls (58%, n = 12, P < 0.05) (Figure 8B).
Figure 8.
Fluorescence angiography of CNV lesions. Fundi of Brown Norway rat eyes were photocoagulated and treated with intravitreal injections of ANP or vehicle as control (A). Representative fluorescein angiograms of animals treated with vehicle or ANP. B: The percentage of lesions graded as 0, I, and IIa, defined as no leakage to moderate leakage, and IIb, considered clinically relevant leakage, in vehicle-treated (n = 20) and ANP-treated animals (n = 18); *P < 0.05.
Discussion
VEGF plays a central role in the pathogenesis of important eye diseases, including AMD and diabetic retinopathy. Inhibition of VEGF has recently become an effective strategy in the treatment of AMD.7,9 However, long-term VEGF inhibition in the eye may be not free of risk, because VEGF also fulfills important physiological functions in the retina and choroidal microvasculature.10 Elucidating the mediators that convey or interfere with VEGF action in vivo will enhance our understanding of the disease and may lead to new molecular therapeutic targets. For instance, recently we characterized azurocidin, an inactive serine protease that is released by leukocytes, as a downstream mediator of VEGF-induced diabetic retinal vascular leakage.19 ANP has been known to interfere with VEGF-induced signaling and permeability in cultured endothelial cells in vitro.16,23 However, the role of ANP in vascular leakage and angiogenesis in vivo was not known.
Our data provide new evidence that ANP suppresses VEGF-induced retinal vascular leakage, both in vitro and in vivo. This suggests a role for ANP and its receptors in the pathogenesis of retinal diseases associated with BRB failure. We show that ANP blocks the effect of VEGF on two retinal cell types, which form the inner and outer BRB within the eye, retinal vascular endothelial and retinal pigment epithelial cells, respectively. The antagonism of VEGF’s action on BRB by ANP is specific as isatin—an NPR antagonist—completely inhibited the observed effect. Our results are in line with previous in vitro studies, showing that ANP blocks the effect of VEGF on endothelial tight junction proteins.23 Although the in vivo results indicate that exogenous ANP prevents VEGF-induced retinal leakage, the signaling pathways in vivo16 or subsequent translocation of tight junction proteins remain to be investigated.
Our data suggest that NP receptors in the RPE are localized in a polarized fashion. ANP blocks the VEGF response, if given apically but not basolaterally, which indicates an apical localization of ANP receptors in the RPE cells. Previous studies have shown that NP receptors are present in the retina13 and in RPE cells24; however, direct evidence for exact localization of the receptors in the retina or RPE cells does not exist.
We show that ANP reduces CNV size in a laser-induced model of proliferative AMD. Under normal conditions, choroidal vasculature is quiescent due to a delicate balance between proangiogenic and antiangiogenic factors. Disturbance of this equipoise, for instance, due to injury or changes in local concentrations of the pro- and antiangiogenic factors in disease or during aging, might lead to the growth of new blood vessels.25 Laser-induced injury in the fundus of rats’ eyes causes a robust neovascularization response, which was potently inhibited in the presence of exogenous ANP. These results indicate that ANP may be regarded as a potent antiangiogenic factor in the eye. Interestingly, ANP is expressed under physiological conditions in ocular tissues and may therefore be an important factor suppressing ocular angiogenesis and vascular leakage in the normal tissue. Furthermore, our FA data show fewer lesions with clinically relevant leakage after ANP administration, supporting the idea that ANP could be used in the treatment of diseases involving retinal leakage.
ANP’s antagonism of VEGF appears unique, because it not only blocks VEGF-induced leakage and angiogenesis but also reduces its production.11,16,23 This study shows the contribution of ANP to BRB breakdown and angiogenesis in an in vivo model of CNV. However, the contribution of ANP to the pathogenesis of AMD remains to be studied. Given that CNV and vascular leakage are serious complications associated with AMD and that ANP is an endogenous antiangiogenic and vascular antipermeability factor, this factor may become useful in the treatment of AMD. Our in vitro data show a relatively extended antagonism of VEGF’s TEER drop when co-incubated with ANP. More surprisingly, ANP’s antagonism of VEGF function also prevails, if the RPE cells had a one-time contact with ANP before being incubated with VEGF, making ANP also a candidate for prevention of leakage.
In summary, the present study is the first to demonstrate that ANP reduces vascular leakage and CNV size in vivo. We show that ANP blocks VEGF-induced vascular leakage in the eye. In addition, we show that ANP reduces the area of CNV in an acute model of AMD. These results suggest ANP as an attractive molecular target in the prevention and treatment of AMD.
Acknowledgments
We thank Miriam Thangaraj and Rebecca C. Garland for the preparation of the manuscript.
Footnotes
Address reprint requests to Ali Hafezi-Moghadam, M.D., Ph.D, 325 Cambridge St., 3rd Floor, Boston, MA 02114. E-mail: AHM@meei.harvard.edu.
Supported by National Institutes of Health grant AI050775 and the National Eye Institute core grants EY14104 and EY14793, and American Health Assistance Foundation, Massachusetts Lions Eye Research Fund, Inc., and Research to Prevent Blindness awarded unrestricted funds to the Departments of Ophthalmology at Harvard Medical School and the Medical University of South Carolina; project also funded by the Marion W. and Edward F. Knight Fund (to A.H-M.).
N.L-C. and S.Z. contributed equally to the work.
References
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Soloway P, Ryan SJ, Miller JW. The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Mol Vis. 1999;5:34. [PubMed] [Google Scholar]
- Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27. [PubMed] [Google Scholar]
- Speicher MA, Danis RP, Criswell M, Pratt L. Pharmacologic therapy for diabetic retinopathy. Expert Opin Emerg Drugs. 2003;8:239–250. doi: 10.1517/14728214.8.1.239. [DOI] [PubMed] [Google Scholar]
- Mechoulam H, Pierce EA. Retinopathy of prematurity: molecular pathology and therapeutic strategies. Am J Pharmacogenomics. 2003;3:261–277. doi: 10.2165/00129785-200303040-00004. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Kourlas H, Abrams P. Ranibizumab for the treatment of neovascular age-related macular degeneration: a review. Clin Ther. 2007;29:1850–1861. doi: 10.1016/j.clinthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Emerson MV, Lauer AK. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs. 2007;21:245–257. doi: 10.2165/00063030-200721040-00005. [DOI] [PubMed] [Google Scholar]
- Emerson MV, Lauer AK, Flaxel CJ, Wilson DJ, Francis PJ, Stout JT, Emerson GG, Schlesinger TK, Nolte SK, Klein ML. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina. 2007;27:439–444. doi: 10.1097/IAE.0b013e31804b3e15. [DOI] [PubMed] [Google Scholar]
- Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D'Amore PA. VEGF and TGF-β are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- Chen HH, Burnett JC., Jr Therapeutic potential for existing and novel forms of natriuretic peptides. Heart Fail Clin. 2006;2:365–373. doi: 10.1016/j.hfc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Rollin R, Mediero A, Roldan-Pallares M, Fernandez-Cruz A, Fernandez-Durango R. Natriuretic peptide system in the human retina. Mol Vis. 2004;10:15–22. [PubMed] [Google Scholar]
- Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989;341:68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Natriuretic peptides suppress vascular endothelial cell growth factor signaling to angiogenesis. Endocrinology. 2001;142:1578–1586. doi: 10.1210/endo.142.4.8099. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor α mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, She H, Nakazawa T, Hisatomi T, Nakao S, Almulki L, Zandi S, Miyahara S, Ito Y, Thomas KL, Garland RC, Miller JW, Gragoudas ES, Mashima Y, Hafezi-Moghadam A. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J. 2008;22:2928–2935. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skondra D, Noda K, Almulki L, Tayyari F, Frimmel S, Nakazawa T, Kim IK, Zandi S, Thomas KL, Miller JW, Gragoudas ES, Hafezi-Moghadam A. Characterization of azurocidin as a permeability factor in the retina: involvement in VEGF-induced and early diabetic blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2008;49:726–731. doi: 10.1167/iovs.07-0405. [DOI] [PubMed] [Google Scholar]
- Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42:789–794. [PubMed] [Google Scholar]
- Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res. 2007;85:762–771. doi: 10.1016/j.exer.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Deciphering vascular endothelial cell growth factor/vascular permeability factor signaling to vascular permeability: inhibition by atrial natriuretic peptide. J Biol Chem. 2002;277:44385–44398. doi: 10.1074/jbc.M202391200. [DOI] [PubMed] [Google Scholar]
- Fujiseki Y, Omori K, Omori K, Mikami Y, Suzukawa J, Okugawa G, Uyama M, Inagaki C. Natriuretic peptide receptors: NPR-A and NPR-B, in cultured rabbit retinal pigment epithelium cells. Jpn J Pharmacol. 1999;79:359–368. doi: 10.1254/jjp.79.359. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Morita I, Tombran-Tink J, Mrazek D, Onodera M, Uetama T, Hayano M, Murota SI, Mochizuki M. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]