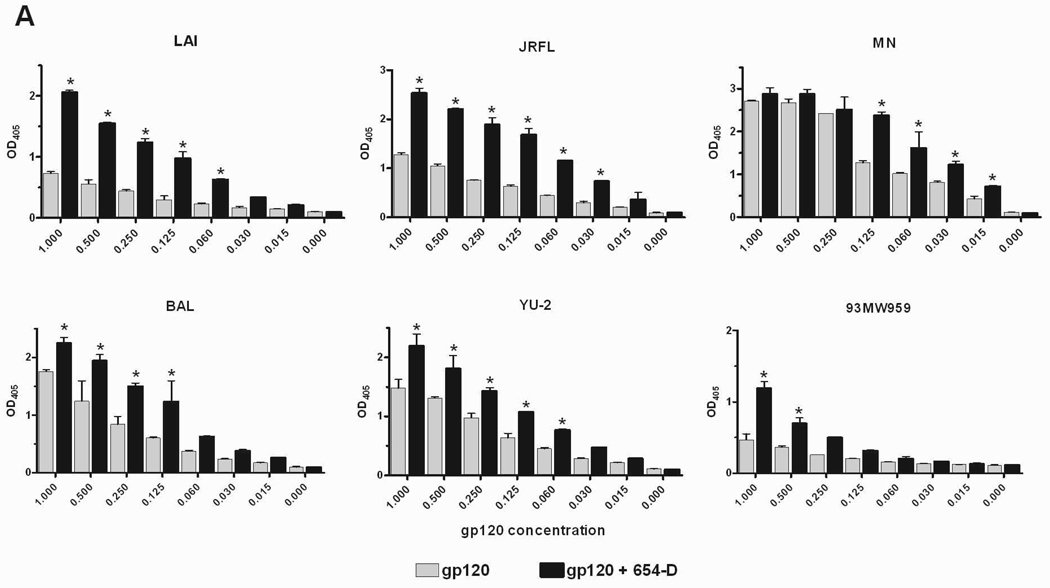
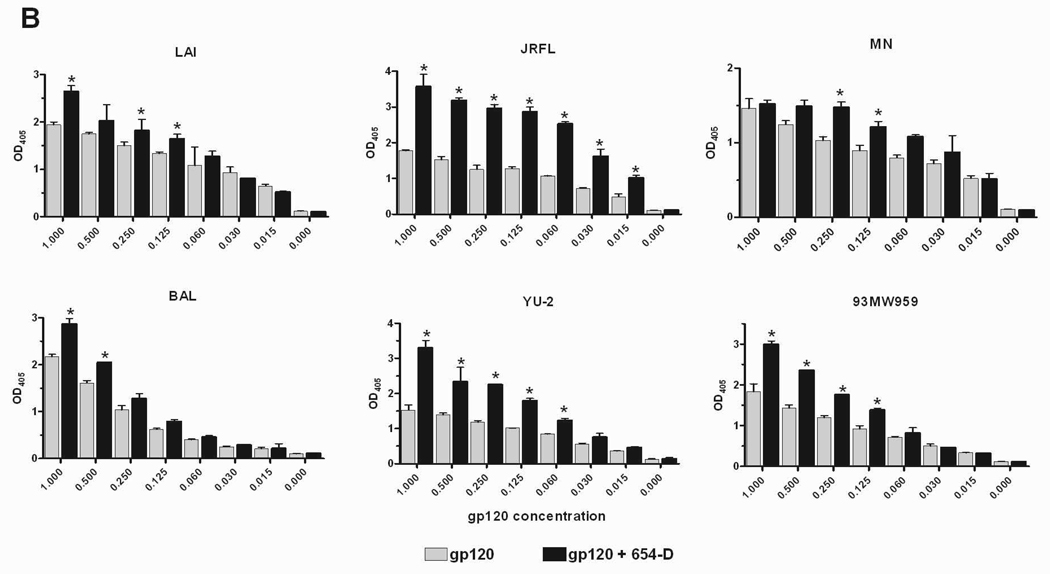
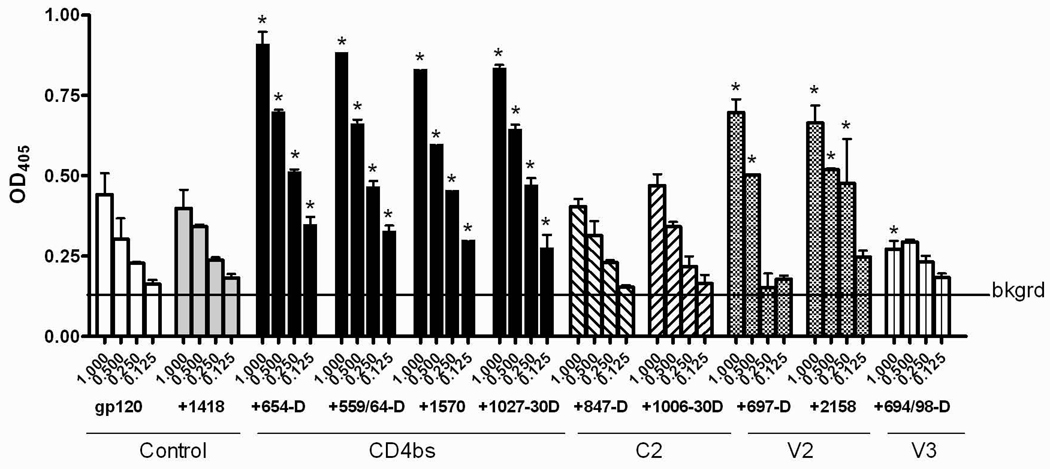
Fig. 2.
Enhanced gp120 antigenicity upon immune complex formation. (A) The binding of anti-V3 mAb to gp120/654-D complexes made of different HIV-1 gp120s. (B) The binding of anti-C1 mAb to gp120/654-D complexes made of different HIV-1 gp120s. (C) The binding of anti-V3 mAb to gp120 complexed with different anti-gp120 mAbs. The gp120/mAb complexes or gp120s alone were coated onto 96-microwell plates and reacted with biotinylated mAbs specific for V3 (694/98-D) (A and C) and C1 (EH21) (B). The binding of the biotinylated mAbs was determined using alkaline phosphatase-conjugated-streptavidin. The x-axis shows equivalent concentrations of gp120, on its own and in the gp120/mAb complexes. Means and standard deviations from duplicate wells are presented in graph. Representative results from one set of experiments are shown. * p<0.05 as compared to gp120 alone. OD405, optical density at 405 nm. Statistical analyses were performed using two-way ANOVA with Dunnett’s multiple comparision test (Graph Pad Prism).