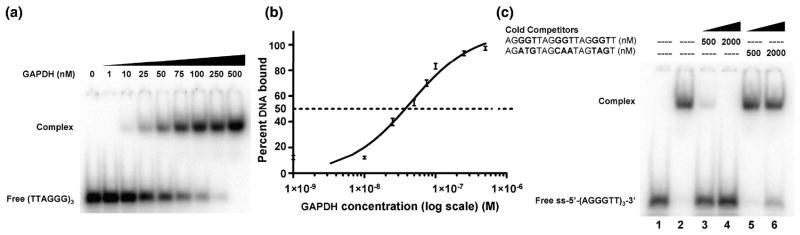
Fig 3.
The binding affinity of GAPDH for a redesigned oligonucleotide of telomeric DNA. (A). An EMSA of the complex formed by titration of 10 pM of [32P]-ss-(AGGGTT)3 into increasing concentrations of GAPDH. (B). Binding isotherm of the data from (A) plotted on a log scale (n=3). The apparent dissociation constant (Kd) is 45 ±5 nM. (C). Increasing concentrations of unlabeled ss-(AGGGTT)3 or control 18mer oligonucleotide (as shown) were incubated with 50 nM GAPDH and 100 nM [32P]-ss-(AGGGTT)3 and analyzed by EMSA as follows: lane 1, free [32P]-(AGGGTT)3; lane 2, [32P]-(AGGGTT)3 and GAPDH; lanes 3 and 4, addition of (unlabeled) ss-(AGGGTT)3 at a concentration of 500 nM (lane 3) and 2 μM (lane 4); lanes 5 & 6, addition of unlabeled control oligonucleotide, also at 500 nM and 2 μM, respectively.