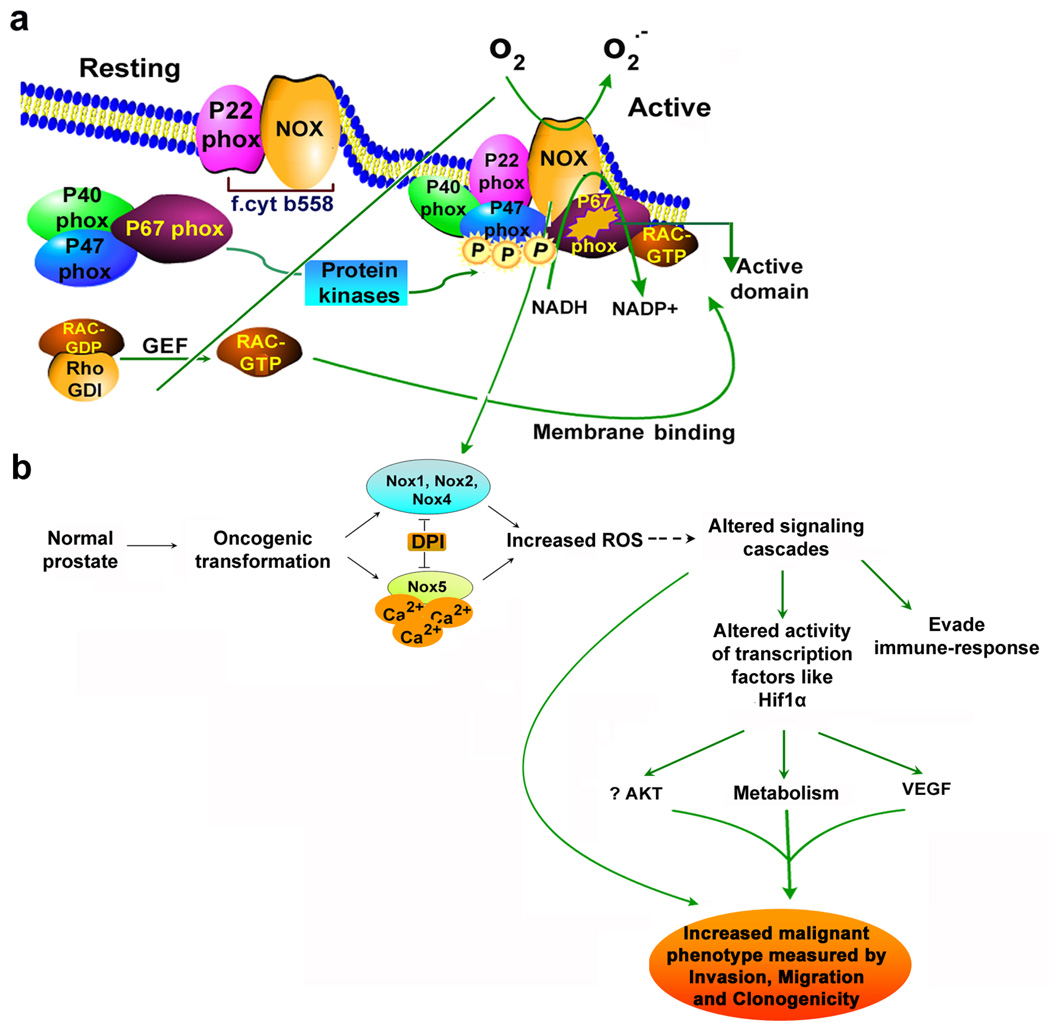
Fig. 3. Activation of reactive oxygen species (ROS) generation by assembly of phox regulatory protein.
(a). Activation of Nox enzyme (equivalent to gp91phox component of phagocytes) system results in assembly of cytosolic regulatory proteins (p40phox, p47phox and p67phox) with flavocytochrome b558 (f.cyt b558; comprised of membrane associated catalytic subunit Nox plus p22phox). These trigger nucleotide exchange protein that activates the GTPase RAC. Protein kinases catalyse many phosphorylation events and allowing p47phox binding to lipid along with p67 phox and p40phox. Activation of exchange factors triggers GTP binding, resulting in conformational change in RAC that promote dissociation from RhoGDI, and promote RAC-GTP binding to p67phox, helping to assemble the active complex. (b). schematic illustrating the role of NAD(P)H oxidase system in prostate cancer cells. In case of prostate cancer cells, NAD(P)H oxidase1 (Nox1), Nox2, Nox3 and Nox4 are similar in size to gp91phox, while Nox5 consists of an additional amino-terminal calcium binding domain, and independent of p22phox requirement for their activity. We hypothesize that increased ROS generation as a result of activation of NAD(P)H oxidase system(s) in prostate cancer cells mediates several signaling pathways critical for growth and could potentially regulate various phenotypic features of cancer cells. Dashed lines indicate possible mechanisms of action.