Abstract
The cholesteryl ester transfer protein (CETP) facilitates the bidirectional transfer of cholesteryl esters and triglycerides (TG) between HDL and (V)LDL. By shifting cholesterol in plasma from HDL to (V)LDL in exchange for VLDL-TG, CETP aggravates atherosclerosis in hyperlipidemic APOE*3-Leiden (E3L) mice. The aim of this study was to investigate the role of CETP in TG metabolism and high-fat diet-induced obesity by using E3L mice with and without the expression of the human CETP gene. On chow, plasma lipid levels were comparable between both male and female E3L and E3L.CETP mice. Further mechanistic studies were performed using male mice. CETP expression increased the level of TG in HDL. CETP did not affect the postprandial plasma TG response or the hepatic VLDL-TG and VLDL-apolipoprotein B production rate. Moreover, CETP did not affect the plasma TG clearance rate or organ-specific TG uptake after infusion of VLDL-like emulsion particles. In line with the absence of an effect of CETP on tissue-specific TG uptake, CETP also did not affect weight gain in response to a high-fat diet. In conclusion, the CETP-induced increase of TG in the HDL fraction of E3L mice is not associated with changes in the production of TG or with tissue-specific clearance of TG from the plasma.
Keywords: cholesteryl ester transfer protein, triglyceride metabolism, HDL, postprandial lipoproteins
Cardiovascular disease (CVD) is one of the major causes of mortality in the Western world, and dyslipidemia is an important risk factor in the development of CVD. Low levels of HDL-cholesterol (HDL-C) and high levels of VLDL cholesterol (VLDL-C), LDL cholesterol (LDL-C), and triglycerides (TG) are independent risk factors for CVD (1, 2). The ratio of (V)LDL-C-HDL-C is to a great extent affected by the cholesteryl ester transfer protein (CETP).
CETP mediates the bidirectional exchange of cholesteryl esters and TG between HDL and (V)LDL. CETP shifts cholesterol in plasma from HDL to (V)LDL and thereby aggravates atherosclerosis in the APOE*3-Leiden (E3L) mouse model, which has a human-like lipoprotein metabolism (3). The HDL-C-lowering effect of CETP has prompted the development of pharmacological CETP inhibitors, such as torcetrapib, as adjuvant therapy to the widely prescribed LDL-lowering statins. Although the first CETP inhibitor torcetrapib recently failed (3, 4), at least two novel CETP inhibitors (i.e. JTT-705 and anacetrapib) are currently in different phases of clinical trials (5, 6).
Despite the well-described effects of CETP on plasma cholesterol metabolism, the role of CETP in TG metabolism has been studied less well. The torcetrapib and JTT-705 trials showed that inhibition of CETP in humans affects both the cholesterol and TG distribution over lipoproteins (7, 8). It was also reported that JTT-705 reduces plasma TG in patients with combined hyperlipidemia (9), which may reveal a beneficial effect of CETP inhibition on plasma TG levels. Furthermore, in rabbits, enrichment of HDL with TG by CETP increases the catabolism of HDL by hepatic lipase (HL) (10). Thus, CETP has the potential to affect TG metabolism, which may have effects on tissue-specific lipid accumulation.
VLDL-derived TG are lipolyzed in peripheral tissues by the enzyme LPL, whereas HDL-derived TG are presumably lipolyzed by HL and thus shunted to the liver. Therefore, we hypothesized that the CETP-mediated net transfer of TG from (V)LDL to HDL modulates the tissue-specific uptake of plasma TG and, as a consequence, affects the development of high-fat diet-induced obesity.
MATERIALS AND METHODS
Animals
Human CETP transgenic mice that express CETP under control of its natural flanking regions (strain 5203) (11) were obtained from Jackson Laboratories (Bar Harbor, ME) and crossbred with E3L mice (12) in our local animal facility to obtain heterozygous E3L.CETP mice (3). Mice (12–16 weeks old) were housed in a temperature- and humidity-controlled environment and were fed a standard chow diet with free access to water. Mice 12 weeks of age were fed a high-fat diet (60% energy derived from bovine fat; D 12492, Research Diet Services, Wijk bij Duurstede, The Netherlands) for 12 weeks to induce obesity. Body weight was measured during the intervention and the delta was calculated. All animal experiments were approved by the Animal Ethics Committee from the Leiden University Medical Center and The Netherlands Organization for Applied Scientific Research, Leiden, The Netherlands.
Plasma parameters
Plasma was obtained after overnight fasting (unless indicated otherwise) via tail vein bleeding in chilled paraoxon-coated capillary tubes to prevent ex vivo lipolysis and assayed for TG and total cholesterol using commercially available kits 1488872 and 236691 from Roche Molecular Biochemicals (Indianapolis, IN), respectively. Plasma CETP mass was analyzed using the CETP ELISA kit from ALPCO Diagnostics (Salem, NH). FFA were measured using NEFA C kit from Wako Diagnostics (Instruchemie, Delfzijl, The Netherlands). HL activity in plasma was determined by measuring plasma triacylglycerol hydrolase activity as described earlier (13).
Lipoprotein profiling
To determine the lipid distribution over plasma lipoproteins, lipoproteins were separated using fast protein liquid chromatography. Plasma was pooled per group, and 50 μl of each pool was injected onto a Superose 6 PC 3.2/30 column (Äkta System, Amersham Pharmacia Biotech, Piscataway, NJ) and eluted at a constant flow rate of 50 μl/min in PBS, 1 mM EDTA, pH 7.4. Fractions of 50 μl were collected and assayed for cholesterol and TG as described above.
Postprandial response
Mice were fasted overnight with food withdrawn at 6:00 PM the day before the experiment. Mice received an intragastric olive oil load (Carbonell, Cordoba, Spain) of 200 µL. Prior to the bolus and 1, 2, 3, 4, 6, and 10 h after the bolus, blood samples (30 µL) were drawn via tail bleeding for TG determination as described above. The circulating levels were corrected for the levels of TG prior to the bolus and the area under the curve (AUC) was calculated over the period of 0–10 h using GraphPad software.
Hepatic VLDL-TG and VLDL-apolipoprotein B production
Mice were fasted for 4 h with food withdrawn at 5:00 AM prior to the start of the experiment. During the experiment, mice were sedated with 6.25 mg/kg acepromazine (Alfasan), 6.25 mg/kg midazolam (Roche), and 0.3125 mg/kg fentanyl (Janssen-Cilag). At t = 0 min, blood was taken via tail bleeding and mice were intravenously injected with 100 µL PBS containing 100 µCi Trans35S label to measure de novo total apolipoprotein B (apoB) synthesis. After 30 min, the animals received 500 mg tyloxapol/kg body weight (Triton WR-1339, Sigma-Aldrich) as a 10% (w/w) solution in sterile saline to prevent systemic lipolysis of newly secreted hepatic VLDL-TG (14). Additional blood samples were taken at t = 15, 30, 60, and 90 min after tyloxapol injection and used for determination of plasma TG concentration. At 120 min, the animals were euthanized and blood was collected by orbital puncture for isolation of VLDL by density gradient ultracentrifugation. 35S-labeled total apoB content was measured in the VLDL fraction after precipitation with isopropanol (15–17).
In vivo clearance of VLDL-like emulsion particles
Glycerol tri[3H]oleate-labeled VLDL-like emulsion particles (80 nm) were prepared as described by Rensen et al. (18). In short, radiolabeled emulsions were obtained by adding 200 µCi of glycerol tri[3H]oleate (triolein, TO) to 100 mg of emulsion lipids before sonication (isotope obtained from GE Healthcare, Little Chalfont, UK). Mice were fasted 4 h, sedated as described above, and injected with the radiolabeled emulsion particles (1.0 mg TG in 200 µL PBS) in the tail vein at 9:00 AM At indicated time points after injection, blood was taken from the tail vein to determine the serum decay of [3H]TO. At 15 min after injection, plasma was collected by orbital puncture and mice were euthanized by cervical dislocation. Organs were harvested and saponified to determine [3H]TO uptake.
Tissue-specific FFA uptake from plasma TG
Mice were fasted for 4 h with food withdrawn at 5:00 AM prior to the start of the experiment. During the experiment, mice were sedated as described above. At t = 0 min, blood was taken via tail bleeding and mice received a continuous intravenous infusion of [3H]TO-labeled VLDL-like emulsion particles for 2 h (4.4 µCi [3H]TO and 1.2 µCi [14C]FA) (19). Blood samples were taken using chilled paraoxon-coated capillaries by tail bleeding at 90 and 120 min of infusion to ensure that steady-state conditions had been reached. Subsequently, mice were euthanized and organs were quickly harvested and snap-frozen in liquid nitrogen. Analysis and calculations were performed as described (19).
Statistical analysis
Differences between groups were determined with the unpaired t-test for normally distributed data (GraphPad Prism 5 software, La Jolla, CA). A P-value < 0.05 was considered statistically significant. Data are presented as means ± SEM.
RESULTS
Plasma lipids, lipoprotein profiles, and HL activity
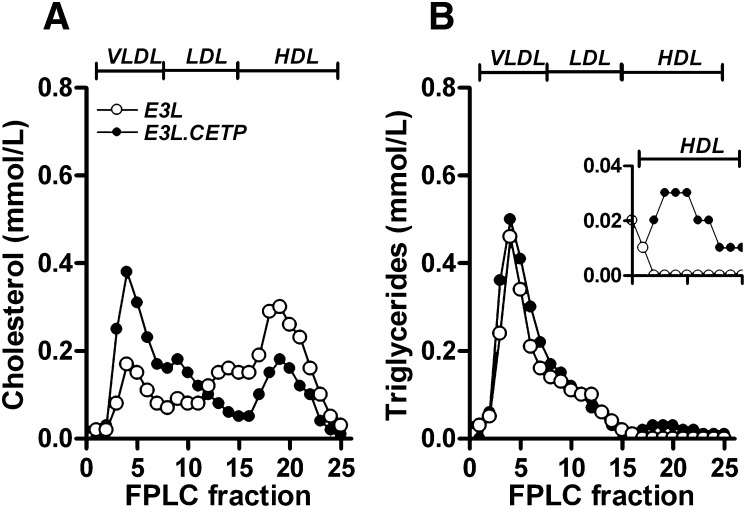
To investigate the role of CETP in TG metabolism, male and female E3L and E3L.CETP mice were fasted overnight and plasma lipid levels were determined (Table 1). Expression of CETP had no effect on total plasma lipid levels. Because we did not detect a difference between males and females, we decided to use only males for all subsequent experiments. Plasma lipoprotein profiles were determined on pooled plasma. Expression of CETP resulted in a shift of cholesterol from HDL to VLDL (Fig. 1A), as seen previously (3, 20). Furthermore, a small amount of TG was detected in the HDL fraction upon expression of CETP (Fig. 1B, insert). HL activity did not differ between E3L and E3L.CETP mice (3.9 ± 1.5 vs. 3.2 ± 1.3 µmol FFA/h/ml, respectively). This indicates that there is exchange of TG from the VLDL to the HDL fraction by CETP, which may be indicative of changes in TG metabolism.
TABLE 1.
Plasma parameters after an overnight fast
| Males |
Females |
|||
|---|---|---|---|---|
| E3L | E3L.CETP | E3L | E3L.CETP | |
| TG (mM) | 2.39 ± 0.13 | 2.45 ± 0.26 | 2.59 ± 0.33 | 2.31 ± 0.46 |
| Total cholesterol (mM) | 3.26 ± 0.11 | 2.91 ± 0.29 | 3.11 ± 0.23 | 3.09 ± 0.45 |
| FFA (mM) | 1.09 ± 0.06 | 1.18 ± 0.07 | 1.18 ± 0.09 | 1.31 ± 0.10 |
| CETP (µg/ml) | n.d. | 3.78 ± 0.36 | n.d. | 3.51 ± 0.43 |
Plasma was obtained from overnight fasted male and female E3L and E3L.CETP mice on a chow diet (n = 12/group). Plasma TG, total cholesterol, FFA, and CETP levels were measured. n.d., not detected.
Fig. 1.
Plasma lipoprotein profiles. The 12 h fasted mice were bled. Plasma was collected, pooled per group (n = 12), and subjected to fast protein liquid chromatography to separate lipoproteins. Distribution of cholesterol (A) and TG (B) over lipoproteins was determined.
Postprandial TG clearance
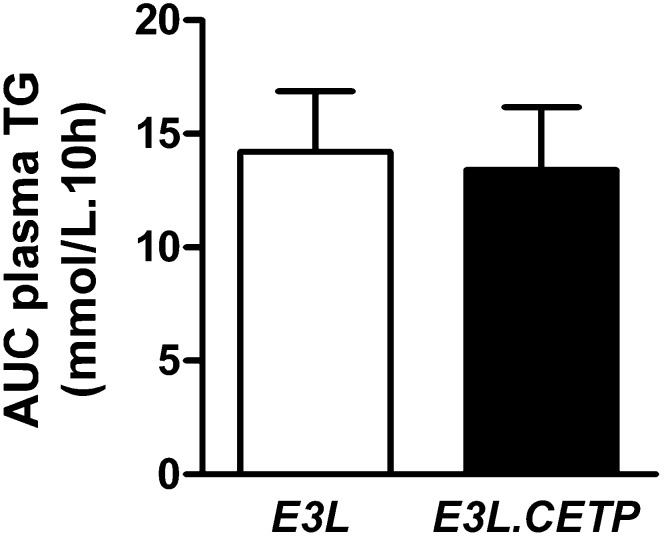
To examine whether CETP-mediated transfer of TG from VLDL to HDL influences plasma TG metabolism, we determined postprandial TG response. After an overnight fast, mice received an intragastric gavage of olive oil. Plasma TG concentrations were measured over a 10 h period and the AUC was calculated. Expression of CETP in E3L mice did not affect the postprandial TG changes in plasma (Fig. 2).
Fig. 2.
Postprandial plasma TG response. Overnight fasted mice received an intragastric olive oil gavage and blood samples were drawn up to 10 h (n = 6–9/group). Plasma TG concentrations were determined and AUC0–10 was calculated.
VLDL-TG and VLDL-apoB production
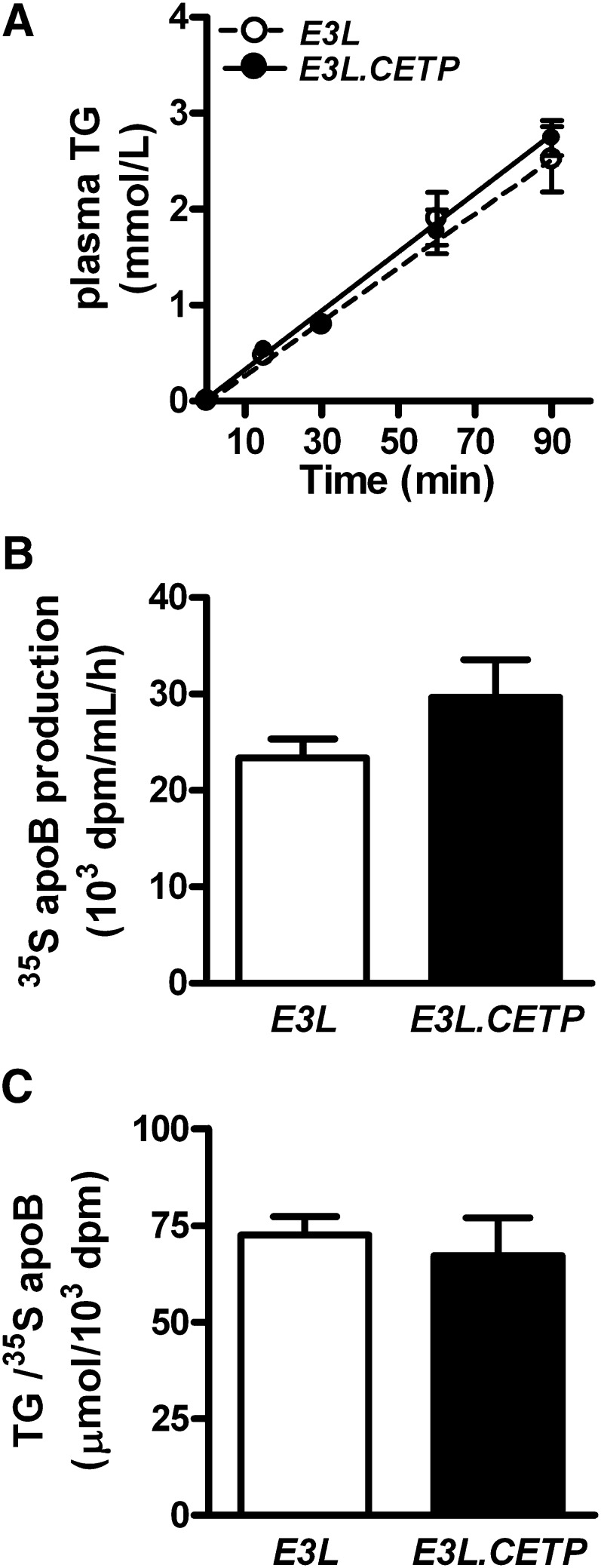
To further examine the effect of CETP on TG metabolism, we determined VLDL production. After 4 h of fasting, mice were injected with Trans35S and tyloxapol and the accumulation of endogenous VLDL-TG in plasma was measured over time. As is evident from Fig. 3A, the VLDL-TG production rate, as determined from the slope of the curve, was unchanged upon expression of CETP. Furthermore, the rate of VLDL-apoB production (Fig. 3B) as well as the ratio of TG-apoB (Fig. 3C), reflecting the amount of TG per VLDL particle, did not differ between E3L and E3L.CETP mice.
Fig. 3.
VLDL-TG and VLDL-apoB production. The 4 h fasted mice were consecutively injected with Trans35S label and tyloxapol and blood samples were drawn up to 90 min (n = 4–6/group). TG concentrations were determined in plasma and plotted as the increase in plasma TG (A). After 120 min, the total VLDL fraction was isolated by ultracentrifugation and the rate of newly synthesized VLDL-apoB (B) and the ratio of TG-apoB (C) was calculated.
VLDL-like emulsion TG clearance
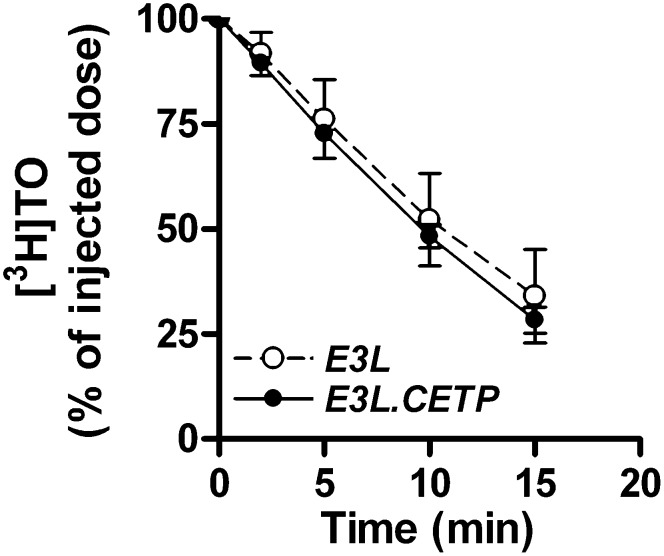
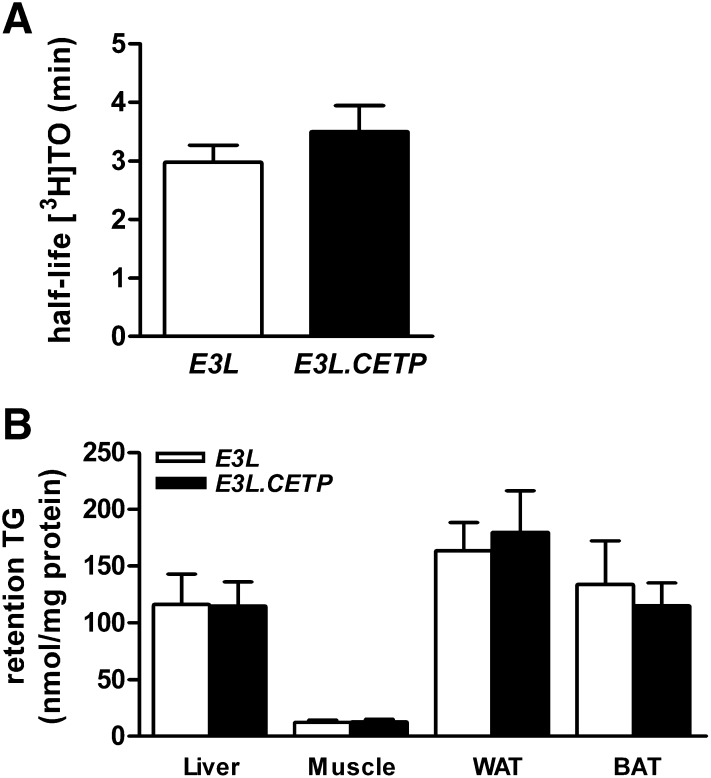
Clearance of TG from circulation is also a major determinant of TG metabolism and therefore we examined the effect of CETP on TG clearance from VLDL-like emulsions, which have previously been shown to mimic the metabolic behavior of TG-rich lipoproteins (18, 21). After 4 h fasting, mice were injected with a bolus of [3H]TO-labeled VLDL-like emulsion particles. The decay of [3H]TO in plasma was not affected by the expression of CETP (Fig. 4). The tissue-specific uptake of [3H]TO was not different between E3L and E3L.CETP mice (data not shown). To determine the body distribution of TG-derived FA in steady state, [3H]TO-labeled VLDL-like emulsion particles together with albumin-bound [14C]FA were continuously infused for 2 h. No difference was observed in the serum half-life of [3H]TO between E3L and E3L.CETP mice (Fig. 5A). Also, the uptake of [3H]TO-derived radioactivity by liver, muscle, white adipose tissue, and brown adipose tissue was not altered due to the expression of CETP (Fig. 5B). The serum half-life and organ-specific uptake of [14C]FA were also not changed upon expression of CETP (data not shown).
Fig. 4.
Plasma TG clearance. The 4 h fasted mice were injected with 1 mg TG as a constituent of VLDL-like [3H]TO-labeled emulsion particles (n = 4–8/group). Blood was collected at the indicated time points and radioactivity was measured in plasma.
Fig. 5.
Plasma-derived TG distribution over tissues. The 4 h fasted mice were infused for 2 h with a trace of VLDL-like [3H]TO-labeled emulsion particles (n = 7/group). Blood and organs were collected. Radioactivity was measured in plasma lipid fractions after thin layer chromatography and the plasma half-life of [3H]TO was calculated (A). The specific [3H]TG activity in plasma was calculated based on the TG level, and the uptake of plasma TG by liver, skeletal muscle, white adipose tissue, and brown adipose tissue was calculated (B).
High-fat diet-induced obesity
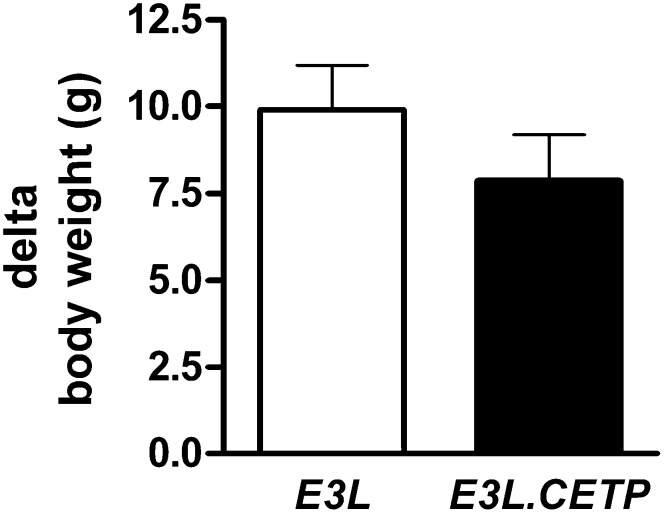
We and others have previously shown that modulation of tissue-specific TG-derived FA delivery can have a major impact on the development of high-fat diet-induced obesity [reviewed in (22)]. To exclude the possibility that CETP expression results in a minor change in tissue-specific TG-derived FA uptake that over a prolonged period would affect the development of obesity, E3L and E3L.CETP mice were fed a high-fat diet (60% energy in the form of fat) for 12 weeks, and body weight was measured over time. The high-fat diet did not affect plasma CETP levels in E3L.CETP mice (3.8 ± 0.4 µg/ml on chow and 3.6 ± 0.3 µg/ml on high-fat diet). Furthermore, the high-fat diet resulted in a similar decrease in plasma TG in both E3L and E3L.CETP mice (1.04 ± 0.11 and 0.92 ± 0.14 mmol/L, respectively). CETP did not affect the high-fat diet-induced body weight gain at any time point during the 12 weeks (Fig. 6).
Fig. 6.
High fat diet-induced obesity. Mice were fed a high-fat diet and body weight was measured during the dietary intervention and the increase in bodyweight was calculated (n = 12/group).
DISCUSSION
Novel drugs that inhibit CETP activity as therapy to increase HDL-C levels are in various stages of development (5, 6). The rationale for development of these drugs is based on the HDL-lowering effect of CETP due to the redistribution of cholesterol from HDL toward (V)LDL. Because CETP transfers both cholesterol and TG between lipoproteins, we focused here on the effect of CETP on TG metabolism. We studied the effect of CETP expression in E3L mice. The E3L mice display a human-like lipoprotein metabolism and are an established model for hyperlipidemia and atherosclerosis [as reviewed in (23)]. We recently reported the HDL-lowering and proatherogenic properties of CETP expression on the E3L background (3). In this study, after 4 h fasting, plasma cholesterol was somewhat higher in the E3L.CETP mice. In the current study, we found no changes in plasma total cholesterol and TG after overnight fasting. We did find a small increase in TG in the HDL fraction upon expression of CETP in E3L mice. Despite this relative increase in HDL-TG, CETP did not affect the postprandial TG response, hepatic VLDL-TG production, clearance of TG from VLDL-like emulsion particles, or the development of high-fat diet-induced obesity. These findings suggest that CETP-mediated transfer of TG from (V)LDL to HDL does not reflect a substantial effect on overall plasma TG metabolism in E3L mice.
There is some controversy regarding the effects of CETP on TG metabolism in various mouse models. Studies in mice, expressing simian CETP, show that on an atherogenic diet, expression of CETP results in increased production and clearance of TG (24). Others have demonstrated that mice expressing human CETP when fed a regular chow diet show no alterations in VLDL-TG production (25, 26). However, Salerno et al. (25) showed that CETP-expressing mice have an increased postprandial TG response and decreased clearance of TG from the circulation. This was attributed to a decrease in LPL activity and LPL gene transcription. We did not find changes in the postprandial TG response or in the clearance of TG from the circulation in E3L.CETP mice versus E3L mice. We also did not find an effect of CETP in E3L mice on high-fat diet-induced obesity. Because modification of tissue-specific FA delivery can significantly affect high-fat diet-induced obesity, this further confirms the absence of even a subtle effect of CETP on tissue-specific TG-derived FA uptake. It seems likely that the explanation for the discrepancy of our data with those of Salerno et al. (25) is associated with the more human-like lipoprotein metabolism on the E3L background as indicated by the presence of a substantial amount of apoB-containing lipoproteins.
Enrichment of HDL with TG has a major impact on HDL metabolism. In humans, it has been demonstrated that cholesterol and apoAI within TG-rich HDL are cleared more rapidly compared with those within TG-poor HDL (27). Similar observations have been made in various animal models (10, 28, 29). Thus, CETP-mediated TG enrichment of HDL has measurable effects on the kinetics of HDL-C and HDL-apoAI. Although these changes in HDL kinetics have the potential to have a substantial effect on TG metabolism, our results indicate that the CETP-mediated TG transfer does not alter the kinetics of TG clearance from the circulation.
This may be explained by the apparently small contribution of HDL-TG to the overall flux of TG. Especially in the postprandial state, the amount of TG in chylomicrons exceeds the amount of TG in HDL by far, even when CETP activity is high. Alternatively, HDL-TG may be readily lipolyzed by HL and the fate of the resulting FA may not be quantitatively different from FA derived from VLDL-TG. During lipolysis of VLDL-TG by LPL, a significant fraction of FA leaks to the circulation and is subsequently cleared by the liver (19). Because it has been postulated that HDL-TG-derived FA are also cleared by the liver (10), the fate of a substantial fraction of VLDL-TG-derived FA and HDL-TG-derived FA will thus be indistinguishable.
In conclusion, we show that expression of CETP does not affect overall TG metabolism and high-fat diet-induced obesity in E3L mice. This implicates that, at least under relatively normolipidemic conditions, pharmacological CETP inhibition is unlikely to disturb TG metabolism.
Acknowledgments
The authors are grateful to M.C. Maas and A.P. Tholens for excellent technical assistance.
Footnotes
Abbreviations:
- apoB
- apolipoprotein B
- AUC
- area under the curve
- CETP
- cholesteryl ester transfer protein
- CVD
- cardiovascular disease
- E3L
- APOE*3-Leiden
- HDL-C
- HDL cholesterol
- HL
- hepatic lipase
- LDL-C
- LDL cholesterol
- TG
- triglycerides
- VLDL-C
- VLDL cholesterol
This work was supported by grants from the Nutrigenomics Consortium/Top Institute Food and Nutrition and the Center of Medical Systems Biology established by The Netherlands Genomics Initiative/Netherlands Organization for Scientific Research and by the Netherlands Organization for Health Care Research Medical Sciences (ZON-MW project no. 948 000 04).
REFERENCES
- 1.Boden W. E. 2000. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High–Density Lipoprotein Intervention Trial. Am. J. Cardiol. 86: 19L–22L. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard B. G., Benn M., Schnohr P., Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298: 299–308. [DOI] [PubMed] [Google Scholar]
- 3.Westerterp M., van der Hoogt C. C., de Haan W., Offerman E. H., Dallinga-Thie G. M., Jukema J. W., Havekes L. M., Rensen P. C. 2006. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler. Thromb. Vasc. Biol. 26: 2552–2559. [DOI] [PubMed] [Google Scholar]
- 4.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield D., Carlson G. L., Sapre A., Tribble D., McKenney J. M., Littlejohn T. W., III, Sisk C. M., Mitchel Y., Pasternak R. C. 2009. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157: 352–360. [DOI] [PubMed] [Google Scholar]
- 6.Rennings A. J., Stalenhoef A. F. 2008. JTT-705: is there still future for a CETP inhibitor after torcetrapib? Expert Opin. Investig. Drugs. 17: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 7.Clark R. W., Sutfin T. A., Ruggeri R. B., Willauer A. T., Sugarman E. D., Magnus-Aryitey G., Cosgrove P. G., Sand T. M., Wester R. T., Williams J. A., et al. 2004. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 24: 490–497. [DOI] [PubMed] [Google Scholar]
- 8.de Grooth G. J., Kuivenhoven J. A., Stalenhoef A. F., de Graaf J., Zwinderman A. H., Posma J. L., van Tol A., Kastelein J. J. 2002. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 105: 2159–2165. [DOI] [PubMed] [Google Scholar]
- 9.Hermann F., Enseleit F., Spieker L. E., Periat D., Sudano I., Hermann M., Corti R., Noll G., Ruschitzka F., Luscher T. F. 2009. Cholesterylestertransfer protein inhibition and endothelial function in type II hyperlipidemia. Thromb. Res. 123: 460–465. [DOI] [PubMed] [Google Scholar]
- 10.Rashid S., Trinh D. K., Uffelman K. D., Cohn J. S., Rader D. J., Lewis G. F. 2003. Expression of human hepatic lipase in the rabbit model preferentially enhances the clearance of triglyceride-enriched versus native high-density lipoprotein apolipoprotein A-I. Circulation. 107: 3066–3072. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X. C., Agellon L. B., Walsh A., Breslow J. L., Tall A. 1992. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J. Clin. Invest. 90: 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Maagdenberg A. M., Hofker M. H., Krimpenfort P. J., de Bruijn I., van Vlijmen B., van der Boom H., Havekes L. M., Frants R. R. 1993. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J. Biol. Chem. 268: 10540–10545. [PubMed] [Google Scholar]
- 13.Berbee J. F., van der Hoogt C. C., Sundararaman D., Havekes L. M., Rensen P. C. 2005. Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J. Lipid Res. 46: 297–306. [DOI] [PubMed] [Google Scholar]
- 14.Aalto-Setala K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. 1992. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietzsch J., Subat S., Nitzsche S., Leonhardt W., Schentke K. U., Hanefeld M. 1995. Very fast ultracentrifugation of serum lipoproteins: influence on lipoprotein separation and composition. Biochim. Biophys. Acta. 1254: 77–88. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Catalina F., Grundy S. M., Patel S. 1996. Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48- relative to B-100-containing lipoproteins. J. Lipid Res. 37: 210–220. [PubMed] [Google Scholar]
- 17.Egusa G., Brady D. W., Grundy S. M., Howard B. V. 1983. Isopropanol precipitation method for the determination of apolipoprotein B specific activity and plasma concentrations during metabolic studies of very low density lipoprotein and low density lipoprotein apolipoprotein B. J. Lipid Res. 24: 1261–1267. [PubMed] [Google Scholar]
- 18.Rensen P. C., Herijgers N., Netscher M. H., Meskers S. C., van Eck M., van Berkel T. J. 1997. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J. Lipid Res. 38: 1070–1084. [PubMed] [Google Scholar]
- 19.Teusink B., Voshol P. J., Dahlmans V. E., Rensen P. C., Pijl H., Romijn J. A., Havekes L. M. 2003. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 52: 614–620. [DOI] [PubMed] [Google Scholar]
- 20.de Vries-van der Weij J., de Haan W., Hu L., Kuif M., Oei H. L., van der Hoorn J. W., Havekes L. M., Princen H. M., Romijn J. A., Smit J. W., et al. 2009. Bexarotene induces dyslipidemia by increased VLDL production and cholesteryl ester transfer protein (CETP)-mediated reduction of HDL. Endocrinology. 150: 2368–2375. [DOI] [PubMed] [Google Scholar]
- 21.Rensen P. C., Jong M. C., van Vark L. C., van der Boom H., Hendriks W. L., van Berkel T. J., Biessen E. A., Havekes L. M. 2000. Apolipoprotein E is resistant to intracellular degradation in vitro and in vivo. Evidence for retroendocytosis. J. Biol. Chem. 275: 8564–8571. [DOI] [PubMed] [Google Scholar]
- 22.Voshol P. J., Rensen P. C., van Dijk K. W., Romijn J. A., Havekes L. M. 2009. Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: studies using genetically engineered mouse models. Biochim. Biophys. Acta. 1791: 479–485. [DOI] [PubMed] [Google Scholar]
- 23.Zadelaar S., Kleemann R., Verschuren L., de Vries-van der Weij J., van der Hoorn J., Princen H. M., Kooistra T. 2007. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 27: 1706–1721. [DOI] [PubMed] [Google Scholar]
- 24.Escola-Gil J. C., Julve J., Marzal-Casacuberta A., Ordonez-Llanos J., Gonzalez-Sastre F., Blanco-Vaca F. 2001. ApoA-II expression in CETP transgenic mice increases VLDL production and impairs VLDL clearance. J. Lipid Res. 42: 241–248. [PubMed] [Google Scholar]
- 25.Salerno A. G., Patricio P. R., Berti J. A., Oliveira H. C. 2009. Cholesteryl ester transfer protein (CETP) increases postprandial triglyceridemia and delays triglyceride plasma clearance in transgenic mice. Biochem. J. 419: 629–634. [DOI] [PubMed] [Google Scholar]
- 26.Harada L. M., Amigo L., Cazita P. M., Salerno A. G., Rigotti A. A., Quintao E. C., Oliveira H. C. 2007. CETP expression enhances liver HDL-cholesteryl ester uptake but does not alter VLDL and biliary lipid secretion. Atherosclerosis. 191: 313–318. [DOI] [PubMed] [Google Scholar]
- 27.Lamarche B., Uffelman K. D., Carpentier A., Cohn J. S., Steiner G., Barrett P. H., Lewis G. F. 1999. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J. Clin. Invest. 103: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchior G. W., Castle C. K., Murray R. W., Blake W. L., Dinh D. M., Marotti K. R. 1994. Apolipoprotein A-I metabolism in cholesteryl ester transfer protein transgenic mice. Insights into the mechanisms responsible for low plasma high density lipoprotein levels. J. Biol. Chem. 269: 8044–8051. [PubMed] [Google Scholar]
- 29.Rashid S., Barrett P. H., Uffelman K. D., Watanabe T., Adeli K., Lewis G. F. 2002. Lipolytically modified triglyceride-enriched HDLs are rapidly cleared from the circulation. Arterioscler. Thromb. Vasc. Biol. 22: 483–487. [DOI] [PubMed] [Google Scholar]