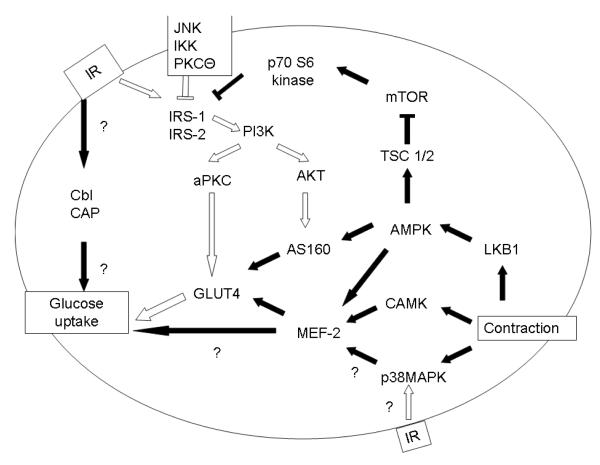
Figure 1.
Glucose uptake in Skeletal Muscle
The insulin receptor is phosphorylated at a tyrosine residue, which then activates tyrosine phosphorylation of IRS-1 and IRS-2. This allows the activation of PI 3-Kinase, which proceeds through either an atypical PKC or AKT mechanism to activate GLUT4 translocation. In insulin resistance (white arrows), decreased tyrosine phosphorylation of the insulin receptor, increased serine/threonine phosphorylation of IRS-1/IRS2, and decreased PI3K activity leads to decreased GLUT4 translocation. IKK, JNK, mTOR and PKCΘ are serine/threonine kinases which act on IRS-1 to inhibit IRS-1 activity. Insulin resistance may also impair p38MAPK response to insulin without affecting p38MAPK response to contraction
Contraction mediates skeletal muscle glucose uptake through activation of p38MAPK, CAMK, and AMPK. Intersection of contraction mediated glucose uptake and insulin mediated glucose uptake include effects at IRS-1, AS160 and p38MAPK.