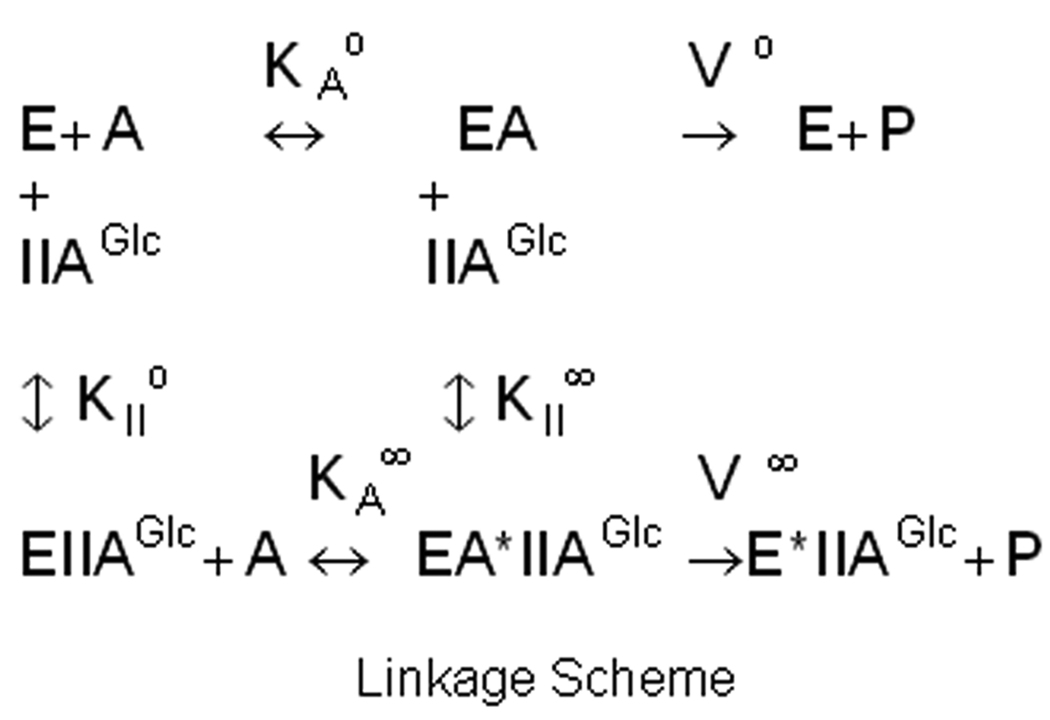
Figure 2.
Thermodynamic linkage scheme for allosteric coupling between IIAGlc and MgATP. E is the EGK-glycerol complex, A is MgATP, KAo is the Michaelis constant for MgATP in the absence of IIAGlc, KA∞ is the Michaelis constant for MgATP in the saturating presence of IIAGlc, KIIo is the dissociation constant for IIAGlc binding in the absence of MgATP, KII∞ is the dissociation constant for IIAGlc binding in the saturating presence of MgATP, Vo is Vmax in the absence of IIAGlc, and V∞ is Vmax in the saturating presence of IIAGlc.