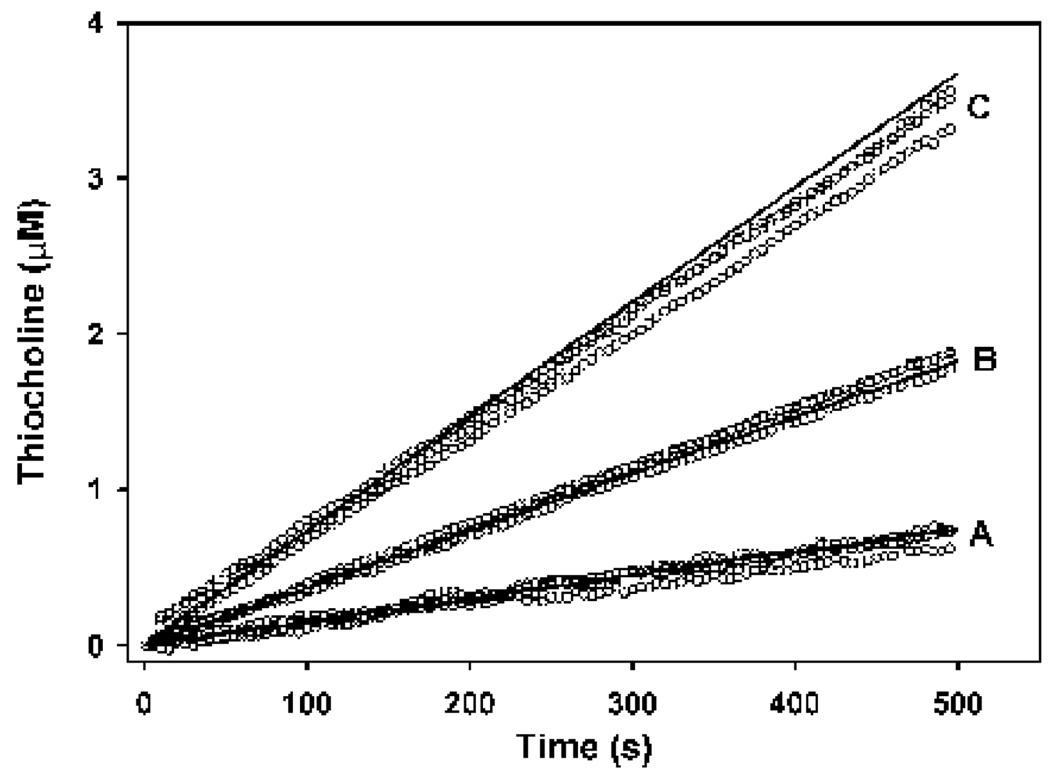
Figure 7. Comparison of butyrylcholinesterase model output (solid lines) at different enzyme and substrate concentrations with empirical data (open circles), with a k1BTC of 0.65 nM−1min−1.
In all cases no chorpyrifos oxon was included. The incubation conditions and model parameters were as follows: butyrylthiocholine = 50 µM with enzyme active sites = 100 pM (A); butyrylthiocholine = 0.25 mM with enzyme active sites = 159 pM (B); butyrylthiocholine = 0.5 mM, with enzyme active sites = 290 pM. The data set in group B were used to optimize the model for k1BTC determinations shown in Figure 6.