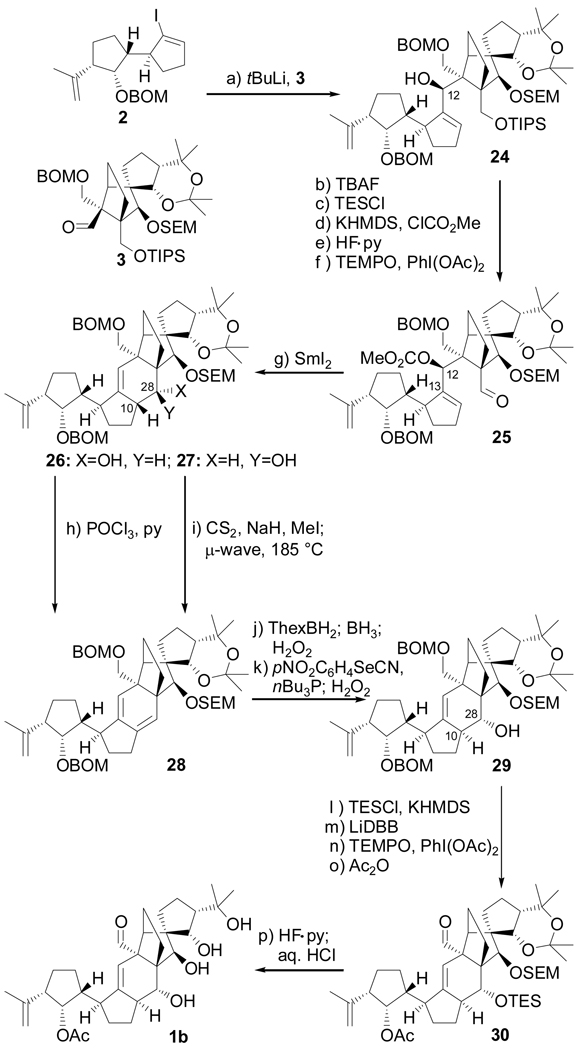
Scheme 3.
Completion of the synthesis of structure 1b. Reagents and conditions: (a) 2 (1.3 equiv), tBuLi (2.6 equiv), THF, −78 → −40 °C, 30 min; then 3 (1.0 equiv), −40 → 0 °C, 20 min, 80 %; (b) TBAF (1.0 M in THF, 5.0 equiv), THF, 25 °C, 1 h, 98 %; (c) TESCl (1.5 equiv), imid (5.0 equiv), CH2Cl2, 25 °C, 1 h, 99 %; (d) KHMDS (0.5 M in PhMe, 3.0 equiv), ClCO2Me (5.0 equiv), Et3N (5.0 equiv), THF, −78 → 25 °C, 2 h; (e) HF•py/py (1:4), 0 → 25 °C, 12 h, 92 % for two steps; (f) TEMPO (1.0 equiv), PhI(OAc)2 (3.0 equiv), CH2Cl2, 25 °C, 24 h, 98 %; (g) SmI2 (0.1 M in THF, 5.0 equiv), HMPA (15 equiv), THF, −10 → 25 °C, 30 min, 80 % (26: 28 %, 27: 52 %); (h) POCl3 (60.0 equiv), py, 60 °C, 3 h, 85 %; (i) CS2 (8.0 equiv), NaH (6.0 equiv), THF, 0 → 25 °C, 30 min; then CH3I (12 equiv), 0 → 25 °C, 3 h; then 185 °C (µ-wave), 1,2-dichlorobenzene, 15 min, 92 %; (j) ThexBH2 (5.0 equiv), THF, −10 → 25 °C, 1 h; then BH3•THF (15 equiv), 0 → 25 °C, 30 min; then 30 % H2O2/3 N NaOH (1:1), 25 → 40 °C, 1 h; 65 % (1:1.3 mix); (k) oNO2C6H4SeCN (2.0 equiv), nBu3P (6.0 equiv), py (12 equiv), THF, 25 °C; then 30 % H2O2, 0 → 25 °C, 67 %; (l) KHMDS (0.5 M in PhMe, 5.0 equiv), TESCl (5.0 equiv), Et3N (8.0 equiv), THF, −78 → 25 °C, 30 min, 94 %; (m) LiDBB (excess), THF, −78 → −50°C, 30 min, 84 %; (n) TEMPO (1.0 equiv), PhI(OAc)2 (3.0 equiv), CH2Cl2, 25 °C, 24 h, 88 %; (o) Ac2O (30 equiv), Et3N (30 equiv), 4-DMAP (1.0 equiv), CH2Cl2, 25 °C, 12 h, 100 %; (p) HF•py/THF (1:4), 25 °C, 3 h; then 3 N aq. HCl/THF (1:3), 25 °C, 6 h, 80 %. KHMDS = potassium hexamethyldisilyazide, TEMPO = 2,2,6,6-teramethyl-1-piperidinyloxy free radical, LiDBB = Lithium ditert- butylbiphenyl.