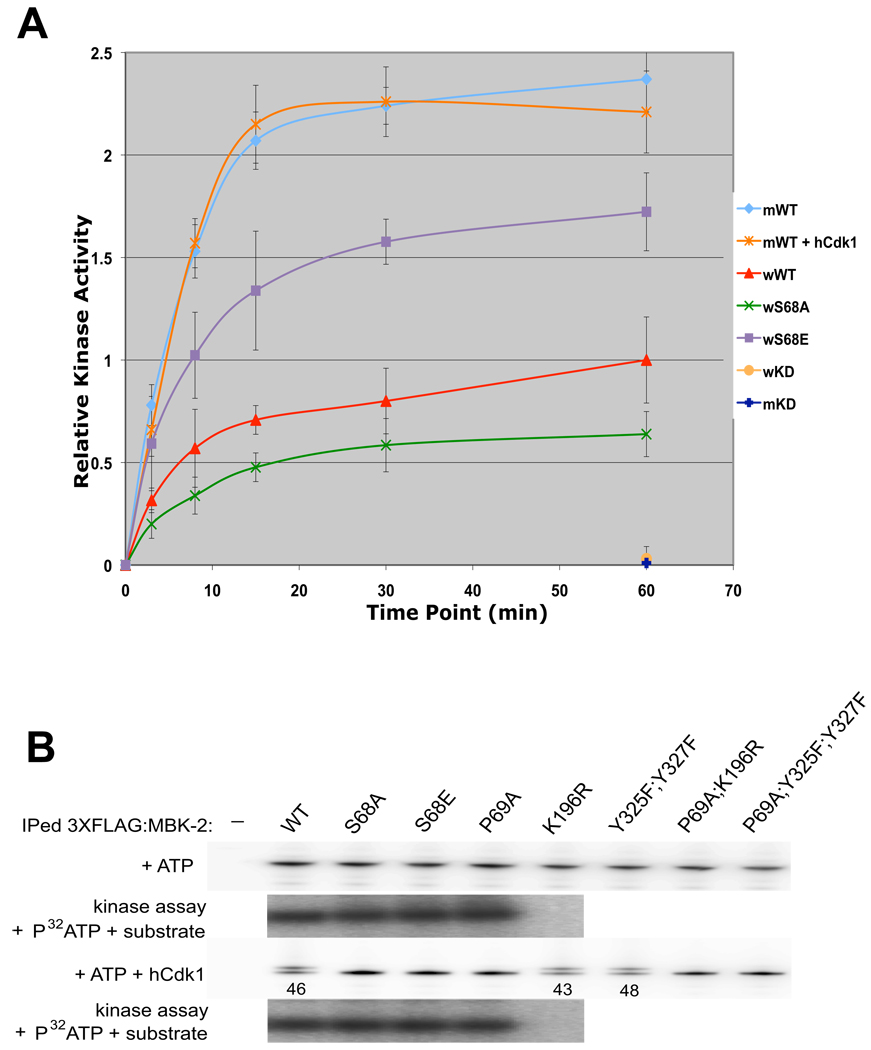
Fig. 3. S68 is required for full kinase activity of MBK-2 synthesized in oocytes, but not of MBK-2 synthesized in mammalian cells.
(A) MBK-2 fusions immunoprecipitated from whole worm extracts (w), or from mammalian cells (m), were treated or not treated with hCdk1, and incubated with recombinant MEI-1(a.a. 51–150) and γ-32P-ATP in kinase buffer for the time shown. Relative kinase activity was calculated by measuring 32P incorporation in MEI-1 expressed as a ratio over the signal obtained for wild-type worm MBK-2 at 60 min. Error bars represent standard deviation from 3 independent immunoprecipitation experiments.
(B) FLAG:MBK-2 fusions immunoprecipitated from mammalian cells were incubated with cold ATP and with or without hCdk1. Half of the samples were run on SDS page (note the presence of slower isoforms in lanes 1, 5 and 6) and the other half was used in a kinase assay again recombinant MEI-1(a.a. 51–150) [no change in kinase activity was detected except in K196R which is not active, as expected (Stitzel et al., 2007)]. Numbers (%) represent the relative amount of phosphorylated FLAG:MBK-2 with respect to total FLAG:MBK-2.