Abstract
Using m-aminophenylboronic acid (APBA) as a functional monomer, molecularly imprinted polymer (MIP) imprinted with bovine serum albumin (BSA) was fabricated on activated glass spheres under optimized conditions. Key factors in the prepolymerization reaction (between APBA and BSA), such as buffer pH, ionic strength and reaction time, were carefully optimized as previously reported [1]. The interaction between APBA and BSA during the prepolymerization stage was investigated and optimized, and ideal conditions for protein rebinding experiments were determined as well. Protein rebinding and enriching properties of polymers were studied in single and competitive binding protocols, respectively. The key point of the present paper is that the binding selectivity of polymers may be estimated by the amount of bound-protein recovered from a protein-saturated polymer. Results demonstrated that the selectivity of MIP towards its template protein is superior to that of non-imprinted polymer (NIP).
Keywords: Molecularly imprinted polymer, m-Aminophenylboronic acid, Protein, Adsorption capacity, Bovine serum albumin (BSA)
1. Introduction
Upon the completion of the International Human Genome Haplotype Map (HapMap) Project, scientists have been engaged in the study of proteomics, which is of great significance for diagnosis and therapy. Proteomics research is composed of two steps: (1) isolation of different protein components from complicated bio-samples (e.g. plasma, urine, saliva, etc.) and (2) biological characterization of individual protein components. However, so far, only the latter can be successfully solved by available approaches. Compositions of bio-samples are very complex. Low-abundance proteins are usually of higher biological significance in diagnosis and therapy than high-abundance species, but it is hard to isolate them from raw bio-samples due to their relatively low representation. As a countermeasure against this, we can remove non-target components out of the system beforehand or enrich the target protein directly. In the present paper, the latter approach was adopted.
Molecular imprinting technology is a high-efficiency technique in the separation field. Previously, MIPs were mainly used for mimicking natural receptors and for the synthesis of polymers carrying specific binding sites with high affinity towards small molecules in organic systems. Currently, we are in great need of environmentally friendly ‘green’ MIPs, which are more compatible with biological systems, particularly in terms of the specific recognition of protein. Consequently, more and more researchers are engaged in exploring alternative polymers, and many positive outcomes have been issued [2-18].
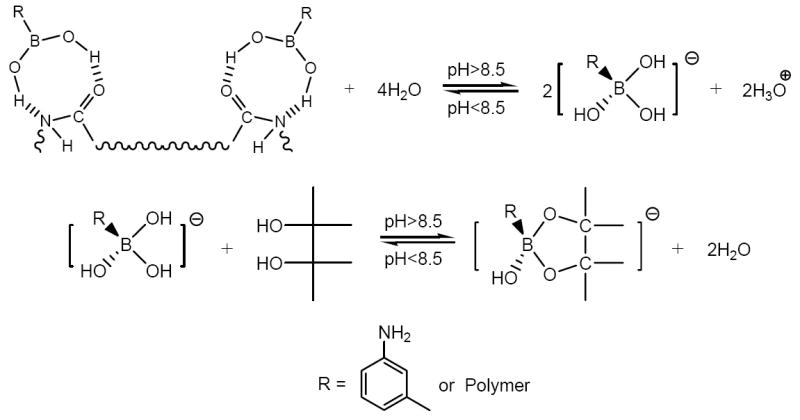
m-Aminophenylboronic acid (APBA) has been widely used in separating saccharin and glycoprotein due to its ability to form reversible covalent bonds with cis-1,2 or cis-1,3-diol containing molecules when the pH is higher than 8.5. In this paper, pH-dependent interactions between polymeric APBA and sorbitol are used to release the trapped template molecules. Recently, APBA has been used as a novel functional monomer for imprinting general proteins [9,15,16]. In this work, to recover bound protein, reversible covalent bonds (between APBA and sorbitol) were substituted for hydrogen bonds (between polymeric APBA and BSA) when the pH ranged from 6.25 to 9.0, ensuring the complete removal of the adsorbed BSA.
In previously published papers, protein-specific imprints were mainly used as sensitive elements on sensors. These sensors are only significant in specific detection, instead of in separation, which they are not capable of doing. In this paper, a strategy based on the classical principle of molecular imprinting technology was updated by the adoption of a promising functional monomer (APBA). By following the previously described method of determining optimal prepolymerization reaction conditions [1], rebinding conditions were chosen as well. Results from protein rebinding and recovery assays indicated that BSA-MIP could selectively bind its template even in protein mixtures and exhibited better specific recognition and enrichment properties than NIP. In a competitive binding assay, the separation factor (β), which will be defined subsequently, was 6.19. Furthermore, the selectivity of polymers was characterized by the amount of bound-protein recovered from the protein-saturated polymer rather than a simple uptake value, in contrast to most other protein imprinting studies. This characterization strategy is of greater significance for component separation in proteomics.
2. Experimental
2.1. Materials
Glass spheres (average particle diameter: 448 μm) were obtained from Hebei Zi-guang Glass Microspheres Corporation (Hebei, China). m-Aminophenylboronic acid (APBA) monohydrate was obtained from Beijing Element Chem.-Tech. Company (Beijing, China). Ammonium persulphate (APS) was obtained from Tianjin North Tianyi factory of chemical reagents (Tianjin, China). Bovine serum albumin, fraction V (BSA, MW 68kDa, pI 4.9) was obtained from Beijing Ding-guo Bio.-Tech. Company (Beijing, China). Lysozyme (MW 14.4kDa, pI 11.0) and bovine haemoglobin (BHb, MW 68kDa, pI 6.8-7.2) were obtained from Shanghai Lan-ji Chem.-Tech. Company (Shanghai, China). All other reagents were obtained from commercial sources. All chemicals were of analytical grade without any fluorescent coexisting substance and were used as supplied without any further purification. Double distilled water was used throughout.
2.2. Optimizing prepolymerization reaction conditions
A fluorescence quenching method was used to investigate the interaction between APBA and BSA. Detailed optimization procedures have been thoroughly described in a previous publication [1]. Briefly, to a 10-mL calibrated test tube 1 mL of 6.0×10-5 M aqueous BSA, a given amount of 6.0×10-5 M aqueous APBA and 0.1 M sodium phosphate buffer solution (pH 6.25) were sequentially added. The mixture was diluted to volume with double distilled water. pH and ionic strength of the system were adjusted to the expected value synchronously, and the solution was mixed thoroughly. Serial solutions of varying concentration of APBA but constant pH, ionic strength and concentration of BSA were prepared. After one hour of incubation at room temperature, fluorescence quenching analysis was carried out using excitation and emission wavelengths of 281nm and 343nm, respectively. Both slit widths of excitation and emission were set at 3 nm.
| (1) |
where F0 and F are fluorescence intensities of one of the above solutions recorded in the absence and presence of APBA, respectively. ΔF is the shift between F0 and F.
Fluorescence of one of the above solutions was strong in the absence of APBA (with maximum emission at 343 nm), while it was significantly quenched with increasing concentration of APBA. Furthermore, Lineweaver-Burk double-reciprocal plots were obtained by plotting 1/ΔF versus 1/[APBA].
2.3. Fabrication of BSA-MIP and NIP on activated glass spheres
Glass spheres were flushed with 1 M NaOH and 1 M HCl in an alternating sequence, with 1 M NaOH for 1 hour, 1 M HCl for another hour, and finally 1 M NaOH again for 1 hour [19]. Before advancing to the next step, bare glass beads were rinsed thoroughly with double distilled water.
To a solution of APBA (100 mM) in 15 mL of 0.04 M phosphate buffer (pH 6.25) was added 35.0 mg of BSA and the mixture was incubated for an hour at room temperature. Upon the addition of 10.00 g activated glass spheres, the mixture was then incubated for a further 2 hours. 20 mL of 100 mM aqueous APS was then slowly admixed with the above solution to initiate the polymerization. Dropwise addition was complete in 20 min. After a 14-hour static polymerization at room temperature, glass spheres coated with BSA-MIP were ready.
NIP was made in a similar manner to that described above, but without the addition of BSA.
The resulting polymer spheres were washed thoroughly with double distilled water and extracted exhaustively with a home-made eluent (0.5 M sorbitol in 0.2 M sodium phosphate buffer, pH 9.0), and again, rinsed thoroughly with double distilled water before use.
2.4. Protein rebinding and recovering assays
The protein rebinding and recovering experiments were performed by using the protocol depicted in Fig. 1. Absorbance of each supernatant at the corresponding wavelength was recorded by UV-vis spectroscopy to determine individual adsorption capacity, which was based on the concentration shift of each protein before and after adsorption (or extraction). Generally, the final equilibrium adsorption capacity (Q) is calculated according to
| (2) |
where C0 is the initial concentration of a protein (mg/mL), Ct is the protein concentration (mg/mL) at a different time, V is the volume of rebinding solution (mL), and W is the weight of the polymer (g).
Fig. 1.
Schematic representation of the protein rebinding and recovering protocols.
In the present paper, concerning bound-protein recovery assays, the final equilibrium adsorption capacity (Q) represents the amount of a particular protein recovered from a protein-saturated polymer. And it is calculated according to
| (3) |
where Ct is the final concentration of a protein in extraction solution(mg/mL), V is the volume of extraction solution (mL), and W is the weight of the polymer (g). In this paper, Qs were estimated using Eq. 3, which is more reasonable and of greater significance than Eq. 2 in protein separation science.
2.4.1 Protein adsorption and extraction assays in single binding protocols
Protein solutions of varying concentration but constant pH and ionic strength were prepared. Two grams of BSA-MIP or NIP were dispersed into a 20 mL solution of one of the three proteins (BSA, lysozyme or BHb). Each sample was incubated for 2 hours at room temperature with agitation (100 rpm) to achieve binding equilibrium.
Upon the complete separation of the two phases (the adsorption solution and the polymer-coated spheres), protein-saturated polymers were washed thoroughly with double distilled water to remove free protein molecules. Subsequently, the polymers were extracted exhaustively with 0.5 M sorbitol in 0.2 M sodium phosphate buffer (pH 9.0). The suspensions were incubated at room temperature with agitation (100 rpm). Two hours later, the amount of recovered protein extracted from a protein-saturated polymer (i.e. final equilibrium adsorption capacity), was calculated using Eq. 3. In this paper, all Qs are means of triplicate experiments.
2.4.2 Protein adsorption and extraction assays in competitive binding protocols
Competitive adsorption experiments were performed using binary protein mixtures of BSA and BHb, which both had a concentration of 0.75 mg/mL in the same solution. Qs were determined by following the approach mentioned above.
3. Results and Discussion
3.1. Survey of optimal conditions of prepolymerization reaction between APBA and BSA
Fluorescence quenching (ΔF) is the shift of fluorescence intensity of BSA at 343 nm caused by the presence of APBA. A stronger interaction between receptor and protein leads to greater fluorescence quenching (ΔF). Thus, through the study of ΔFs, optimal prepolymerization conditions (such as the pH, ionic strength and reaction time) as well as prepolymerization reaction extent can be determined. Accordingly, in a new molecular imprinting system, the analysis of ΔFs can provide a valuable tool for a rapid investigation of optimal prepolymerization reaction (between a functional monomer and template) conditions.
The data obtained from ΔF values provided a good linear relationship. Plotting (1/ΔF)BSA versus (1/[APBA])) indicates that the self-assembly between them induces a single static fluorescence quenching of BSA [1]. Higher ΔF is attributed to greater complex stability. Furthermore, greater complex stability might yield polymers with greater selectivity and higher adsorption capacity, due to better defined recognition sites. Presumably, functional group orientation and even total net charge of recognition sites in MIP created under such optimal reaction conditions could match the conformation of the template (BSA) very well. So it is supposed that if protein rebinding conditions were made identical to prepolymerization reaction conditions, greater selectivity and higher adsorption capacity of MIP towards its template could be achieved as well. Accordingly, the previously determined optimal prepolymerization reaction conditions were adopted in the protein rebinding step as well to improve the template recognition ability of MIP.
3.1.1 Effect of pH
Further experiments have shown that the pH of the prepolymerization reaction and protein rebinding systems should be controlled at 6.25 as previously reported [1].
The isoelectric point (pI) of BSA is about 4.9. When the system is buffered at pH 6.25, a BSA molecule has a net negative charge on its surface, while polymeric APBA is positively charged [16]. Throughout the protein binding process, BSA is adsorbed at the polymer/solution interface due to electrostatic force between BSA-MIP and BSA molecules. Since polymeric APBA has a net negative charge when pH is higher than 8.5 [20], the electrostatic repulsion between polymeric APBA and BSA hinders their binding interaction. Therefore, the pH of the system seems to play an important role via the binding of heterogeneously charged species and repulsion of homogeneously charged ones.
The imprinting effect of a polymer can be described as the imprinting factor and denoted as α.
| (4) |
where QMIP and QNIP are the final equilibrium adsorption capacities of the MIP and NIP for the same protein, respectively. Controlling pH of the synthesis system at 9, Q of the resulting BSA-MIP (towards BSA) is lower than that of BSA-MIP (towards BSA) formed at pH 6.25. However, this trend was reversed in NIP. It is implied that the template adsorption selectivity of polymers synthesized at pH 6.25 (α = 1.18) is much better than those formed at pH 9.0 (α = 0.58). The result confirms the effect of pH on binding as mentioned above.
3.1.2 Effect of ionic strength
Ionic strength during polymer synthesis is an important factor in later protein binding process. As shown in Fig. 2, α is 6.17 for the polymers synthesized at a lower ionic strength (25.3 mmol/kg). Although the BSA Qs of the above polymers (MIP and NIP) are a little bit worse than equivalent polymers fabricated at a higher ionic strength (50.7 mmol/kg), the selectivity is much better, since the α value corresponding to a higher ionic strength is 1.18. Polymeric APBA is positively charged in contrast to BSA at pH 6.25 [16]. A higher concentration phosphate buffer resulted in weakening of the selective binding of protein to the polymer, probably due to the shielding effect of negative ions [21]. Accordingly, in order to improve template binding selectivity of MIPs, polymers must be synthesized at a lower ionic strength, and a low ionic strength in the protein rebinding system will improve results.
Fig. 2.
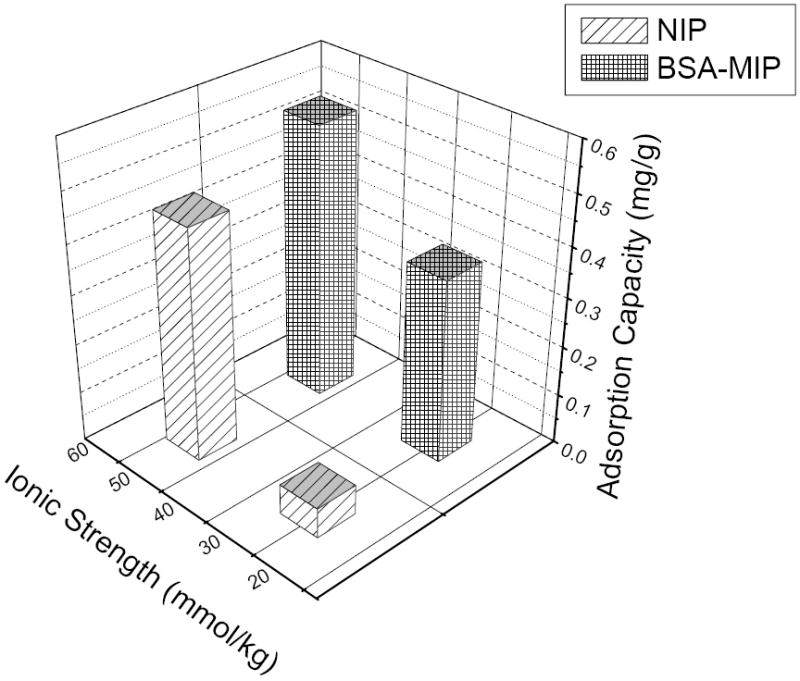
Influence of ionic strength on protein rebinding selectivity of polymers in rebinding BSA.
Results of fluorescence quenching showed that self-assembly between APBA and BSA confirmed the predictions presented in Fig. 1.
3.2. Imprinting efficiency of BSA-MIP
3.2.1 Single binding assay (lysozyme and BHb served as control proteins)
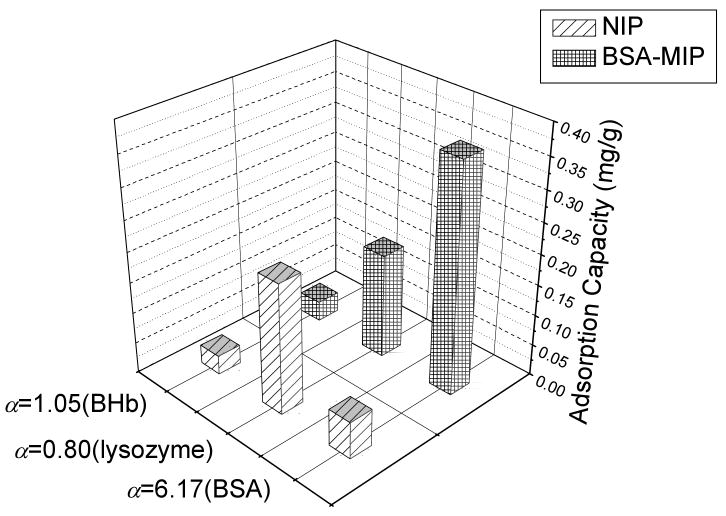
Fig. 3 shows individual Qs of various protein species in BSA-MIP or NIP. In contrast to NIP, BSA-MIP reveals a significantly higher affinity towards its target protein BSA (αBSA = 6.17) over other species examined. Lysozyme and BHb are both non-template proteins. Either BSA-MIP or NIP has no binding selectivity for either, which is revealed by αlysozyme (0.80) and αBHb (1.05). From the molecular level point of view, the three proteins are different in size-scale and functional group orientation. BSA and BHb molecules are similar in size-scale, while lysozyme is the smallest. Since different in amino-acid sequences, the three proteins are distinguished in functional group orientation.
Fig. 3.
Final equilibrium adsorption capacities of BSA-MIP and NIP in single (BSA, Lysozyme and BHb) protein-binding protocols.
In the present protein binding system, BSA-MIP bears cavities complementary in shape and functional group orientation to the target molecules [22]. Since the recognition cavities in BSA-MIP were created by BSA, the final equilibrium adsorption capacity of BSA in BSA-MIP is much higher than that of the control proteins. Lysozyme molecules are small enough to easily access the binding sites, although thus formed “Q” is independent of imprinting. Presumably, multiple hydrogen bonds were randomly formed between lysozyme and polymers. As a result of the accessible surface area for binding, the Qlysozyme in BSA-MIP is higher than that of the other control protein (BHb). For two proteins of similar size, such as BSA and BHb, surface charge and functional group orientation are instead used to manipulate the protein binding property simultaneously. Without charge-charge interactions and configuration matching between protein and polymer networks, an adsorption candidate will poorly bind to the polymers due to the lack of usable binding area. Although the molecular weight and size of BHb are similar to BSA, without compatibility in shape and functional group orientation, the cavities generated by BSA in BSA-MIP are almost inaccessible to BHb, which as a result has the lowest Q in BSA-MIP.
As for NIP, the interactions between protein molecules and polymer networks are random. The binding capacities of various proteins in NIP are very similar, since NIP has no selectivity for any protein.
3.2.2 Competitive binding assay (BSA/BHb)
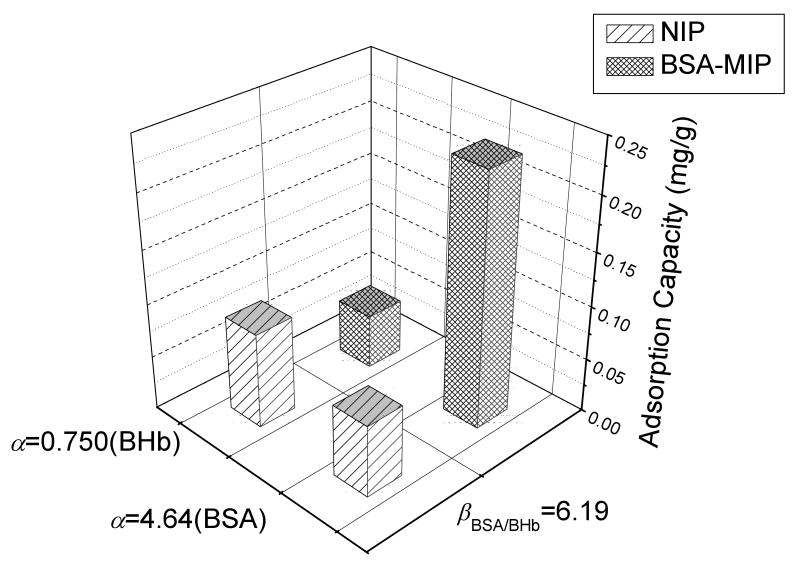
Recognition specificity is evaluated by the separation factor (β), where β is defined as follows
| (5) |
the αtemplate and αcompetitor are the imprinting factors of the template (BSA) and competitor (BHb), respectively. As presented in Fig. 4, in the competitive binding assay, BSA-MIP is able to bind 0.23 mg/g of BSA versus 0.05 mg/g of the competitor (BHb). Recognition sites in the BSA-MIP preferentially adsorb BSA rather than BHb due to the compatibility between recognition sites and BSA molecules. In this work, β is 6.19, which is a high rank in analogous protein imprinting formats. It is demonstrated that BSA-MIP is superior to NIP in binding selectivity as well as specificity.
Fig. 4.
Final equilibrium adsorption capacities of BSA-MIP and NIP in competitive (BSA/BHb) protein-binding protocols.
Adsorption capacity of bare glass beads for any of the three proteins is undetectable due to their non-porous nature.
We can conclude that MIP fabricated using this method is promising for protein separation science.
4. Conclusions
A highly efficient BSA-MIP was generated by using BSA together with a novel functional monomer APBA. Optimal prepolymerization reaction conditions were adopted in order to improve the binding selectivity of BSA-MIP. It is suggested that the specific recognition not only depends on the hydrophobic interactions, but also on compatibility in the sense of the functional group orientation and/or surface charge between the binding sites and protein molecules. BSA-MIP synthesized using this method shows good template selectivity both in single and competitive protein-binding assays. The observed Qs of MIP are lower than those of some other MIPs synthesized with analogous approaches [5,13], but the current MIP has an improved imprinting factor α and a relatively high separation factor β. Key to the present paper is that the adsorption capacities were evaluated by the amount of bound-protein recovered from the protein-saturated polymers, which is significant for protein isolation. The MIP synthesis strategy and the novel functional monomer are promising for separating and purifying specific protein compositions in bio-samples.
Acknowledgments
The project is supported by the National Basic Research Program of China (973 program, grant No.: 2007CB914101).
List of Symbols
- APBA
m-Aminophenylboronic Acid
- MIP
Molecularly Imprinted Polymer
- NIP
Non-imprinted Polymer
- BSA
Bovine Serum Albumin
- BHb
Bovine Haemoglobin
- α
Imprinting Factor
- β
Separation Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang HF, Li WY, He XW, et al. Acta Chimica Sinica. 2007;65:43–48. [Google Scholar]
- 2.Nishino H, Huang CS, Shea KJ. Angew Chem Int Ed. 2006;45:2392–2396. doi: 10.1002/anie.200503760. [DOI] [PubMed] [Google Scholar]
- 3.Hirayama K, Burow M, Morikawa Y, et al. Chem Lett. 1998:731–732. [Google Scholar]
- 4.Pang XS, Cheng GX, Li RS, et al. Anal Chim Acta. 2005;550:13–17. [Google Scholar]
- 5.Guo TY, Xia YQ, Hao GJ, et al. Carbohydr Polym. 2005;62:214–221. [Google Scholar]
- 6.Guo TY, Xia YQ, Wang J, et al. Biomaterials. 2005;26:5737–5745. doi: 10.1016/j.biomaterials.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Chou PC, Rick J, Chou TC. Anal Chim Acta. 2005;542:20–25. [Google Scholar]
- 8.Rick J, Chou TC. Biosens Bioelectron. 2005;20:1878–1883. doi: 10.1016/j.bios.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Rick J, Chou TC. Anal Chim Acta. 2005;542:26–31. [Google Scholar]
- 10.Hawkins DM, Stevenson D, Reddy SM. Anal Chim Acta. 2005;542:61–65. [Google Scholar]
- 11.Li Y, Yang HH, You QH, Zhuang ZX, Wang XR. Anal Chem. 2006;78:317–320. doi: 10.1021/ac050802i. [DOI] [PubMed] [Google Scholar]
- 12.Shiomi T, Matsui M, Mizukami F, et al. Biomaterials. 2005;26:5564–5571. doi: 10.1016/j.biomaterials.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Guo TY, Xia YQ, Hao GJ, et al. Biomaterials. 2004;25:5905–5912. doi: 10.1016/j.biomaterials.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Rezeli M, Kilár F, Hjertén S. J Chromatogr A. 2006;1109:100–102. doi: 10.1016/j.chroma.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 15.Piletsky SA, Piletska EV, Bossi A, et al. Biosens Bioelectron. 2001;16:701–707. doi: 10.1016/s0956-5663(01)00234-2. [DOI] [PubMed] [Google Scholar]
- 16.Bossi A, Piletsky SA, Piletska EV, et al. Anal Chem. 2001;73:5281–5286. doi: 10.1021/ac0006526. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama K, Sakai Y, Kameoka K. J Appl Polym Sci. 2001;81:3378–3387. [Google Scholar]
- 18.Huang JT, Zhang J, Zhang JQ, et al. J Appl Polym Sci. 2005;95:358–361. [Google Scholar]
- 19.Bossi A, Castelletti L, Piletsky SA, Turner APF, et al. J of Chromatogr A. 2004;1023:297–303. doi: 10.1016/j.chroma.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Asher SA, Alexeev VL, Goponenko AV, et al. J Am Chem Soc. 2003;125:3322–3329. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 21.Hianik T, Ostatná V, Sonlajtnerova M, et al. Bioelectrochem. 2007;70:127–133. doi: 10.1016/j.bioelechem.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Peppas NA, Huang YB. Pharm Res. 2002;19:578–587. doi: 10.1023/a:1015389609344. [DOI] [PubMed] [Google Scholar]