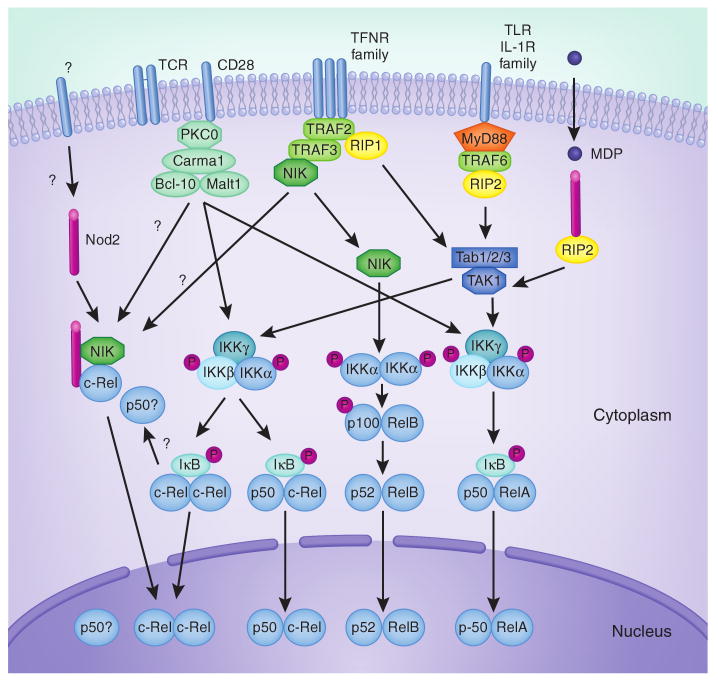
Figure 1.
Possible cross-talk between Nod2, TCR, Cd28, TLR and TNFR family members in regulating NF-κB. In non-T cells, muramyl dipeptide (MDP) can initiate a signaling cascade that requires Nod2 and RIP2, and results in nuclear accumulation of p50 and RelA as a result of Tab-TAK1-mediated activation of the IKKα-IKKβ-IKKγ complex and subsequent degradation of IκB. This pathway is also activated by TLR and IL-1R family members through MyD88, TRAF6 and RIP2, and by TNFR family members through TRAF2 and RIP1. whereas TLR, IL-1R and TNFR members can promote p50-RelA activation in T cells, it is not clear whether MDP, through Nod2, can also affect this pathway in T cells or whether T cells can take up MDP through specific transporters or phagocytosis. Shaw et al.3 propose that Nod2, independent of MDP recognition, influences T cell activity by binding NIK and c-Rel and by promoting c-Rel nuclear accumulation (left). whether c-Rel is activated as a homodimer or as a heterodimer with p50 was not investigated. It is unclear whether this putative NIK–c-Rel–Nod2 complex is downstream of the TCR, CD28, cytokine receptors or other receptors, and whether it is associated with other molecules. Cooperation between the TCR and CD28 in T cells can also promote p50-RelA nuclear translocation through PKCθ-Carma1-Bcl10-Malt1. TCR, CD28, TNFRs and TLRs can also trigger nuclear translocation of c-Rel (in the form of c-Rel–c-Rel or p50–c-Rel dimers) by targeting IκB. TNFR family members, by recruiting TRAF3 and thus inhibiting TRAF3-mediated degradation of NIK, can also activate the IKKα-dependent noncanonical NF-κB pathway leading to p52-RelB nuclear translocation. This may also promote cross-talk with Nod2 by stabilizing and freeing NIK to allow formation of the NIK–c-Rel–Nod2 complex.