Fig. 9.
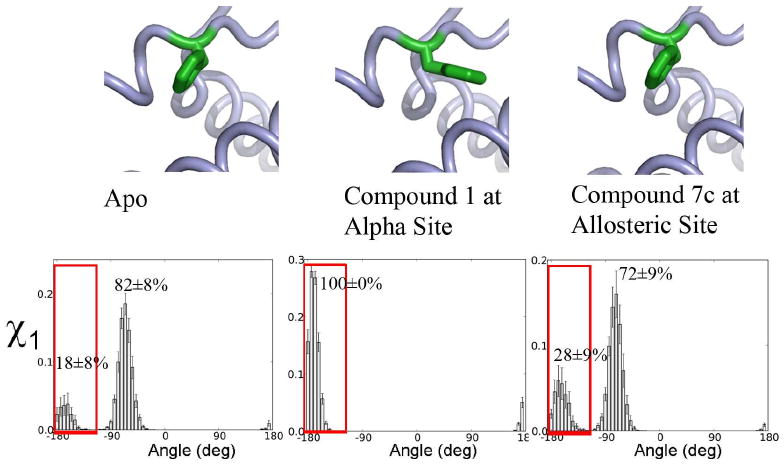
Compound binding to the allosteric site causes a population shift in the conformation of hot-spot residue Phe42 that favors binding compound at the IL-2Rα-competitive site. (Top) Conformations of Phe42 in apo and compound-bound crystal structures (PDB IDs 1M47, 1M48, and 1NBP, resp.). (Bottom) Histograms of Phe42's χ1 angle from MD simulations. Red boxes highlight the χ1 population selected by ligand binding at the competitive site.
