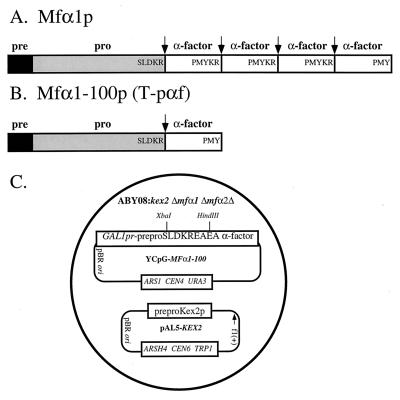
Figure 1.
Schematic structures of α-factor precursors and overview of the genetic system. (A) Signal peptidase cleavage of the major form of prepro-α-factor (165 residues), which is encoded by the MFα1 gene, results in production of 146 residue pro-α-factor (18). Kex2 protease cleaves pro-α-factor between the glycosylated “pro” domain and the first α-factor repeat carboxyl to the sequence Ser-Leu-Asp-Lys-Arg↓ and at three sites between the α-factor repeats carboxyl to the sequence Pro-Met-Tyr-Lys-Arg↓ (19). Production of mature α-factor requires exoproteolysis by Kex1 carboxypeptidase to remove C-terminal Lys and Arg residues from the internal repeats (20) and by Ste13 dipeptidyl aminopeptidase to remove N-terminal GluAla and AspAla dipeptides from each repeat (21). (B) The product of the MFα1–100 gene contains a single α-factor repeat. Production of mature α-factor requires cleavage by Kex2 at a single site. (C) Schematic of yeast strain ABY08 carrying plasmids YCpG-MFα1–100 and pAL5-KEX2. The Xba I and HindIII sites in YCpG-MFα1–100 were used for cassette mutagenesis of the P2 Lys (Lys84). An important feature of this system is that cleavage of both wild-type and mutant sites must occur in the correct “phase,” i.e., immediately following the P1 Arg. This is because production of mature α-factor requires removal of two Glu-Ala dipeptides by the Ste13 peptidase.