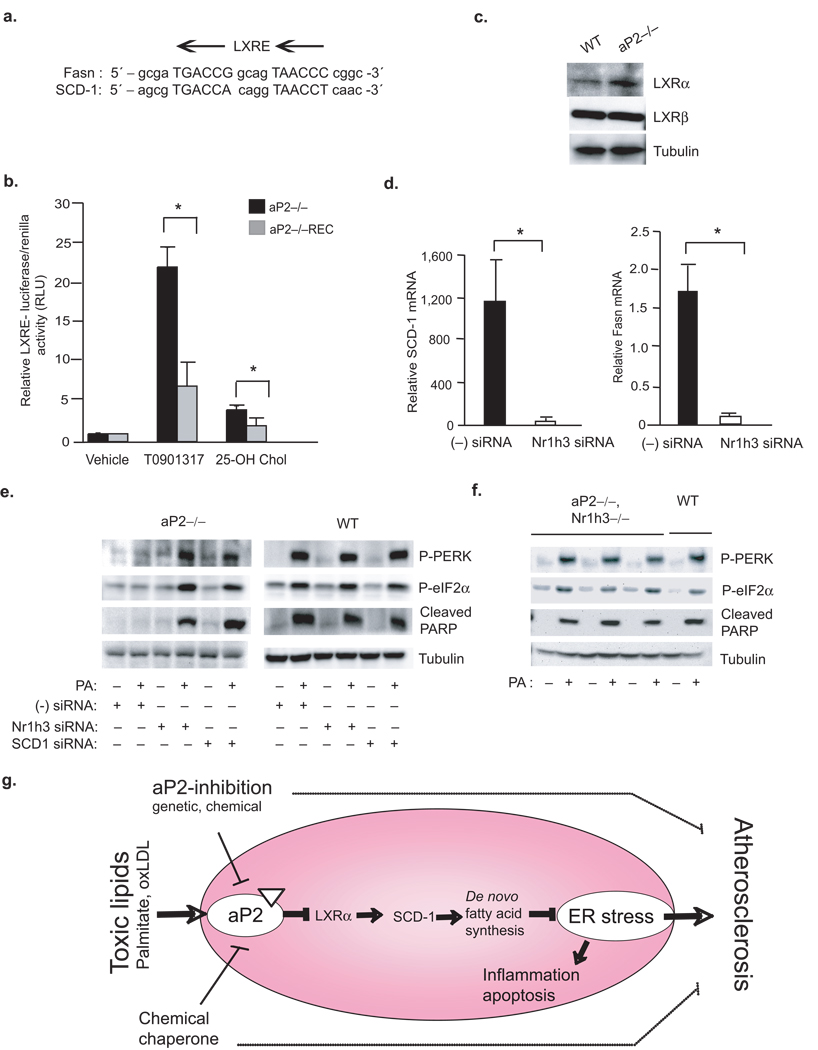
Figure 6. Linking toxic lipids to ER stress and atherosclerosis through aP2-LXR–α crosstalk.
(a) Alignment of LXR responsive element (LXRE) on Fasn and SCD-1 promoters. (b) LXR–driven transcriptional activity was determined from various macrophage lines upon stimulation with a synthetic T0901317 (10 µM) or endogenous 25-hydroxycholesterol (10 µM) LXR ligand (luciferase activity is reported after normalizing to transfection efficiency). (c) Relative LXR–α and LXR–β protein levels in various macrophage lines were examined by Western blotting (d) Relative SCD-1 and Fasn mRNA levels from aP2−/− macrophage treated with a scrambled (−) or Nr1h3-specific siRNA were examined by qRT-PCR. (e) Lysates from various macrophages treated with scrambled (−) siRNA or a specific siRNA against SCD-1 or Nr1h3 and stressed with or without PA (500 µM) were examined for P-PERK and P-eIF2–α by Western blotting. (f) Lysates from peritoneal macrophages from aP2−/−Nr1h3−/− or WT mice stressed with or without PA (500 µM) were examined for P-PERK, P-eIF2–α and cleaved PARP by Western blotting. (g) A cellular lipotoxicity model: Toxic levels of lipids are sensed by the ER through an aP2-dependent pathway and induce the UPR and lead to macrophage apoptosis. The absence of aP2 serves to reactivate macrophage de novo lipogenesis pathways and promotes desaturation, particularly through LXRα-mediated activation of SCD-1, leading to increased production of bioactive lipids and resistance to ER stress. Our findings indicate that alleviation of macrophage ER stress, either through aP2 inhibition or enhancing ER function, is protective against atherosclerosis.