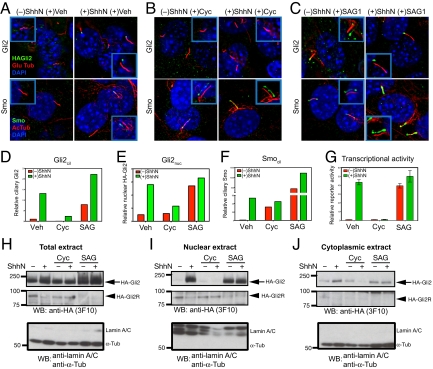
Fig. 2.
Gli2 accumulate at the primary cilium and the nucleus upon Hh stimulation, and pharmacological manipulation of Smo activity in cilia affects ciliary and nuclear accumulation and transcriptional activity of Gli2. (A–F) NIH 3T3/HA-Gli2 cells were incubated with or without ShhN in the presence of vehicle control (Veh), cyclopamine (3 μM) or SAG1 (400 nM) for 24 h. Cells were then stained to visualize Gli2, Smo, the primary cilium, and the nucleus as indicated. The Insets show shifted overlays. (A) ShhN-induced accumulation of Gli2 and Smo at the primary cilium. NIH 3T3/HA-Gli2 cells were grown to confluency and incubated in the presence or absence of ShhN; cells were stained to visualize HA-Gli2, the primary cilium (Glu tubulin), and the nucleus (DAPI) in Upper panels, or Smo, the primary cilium (acetylated tubulin), and the nucleus (DAPI) in Lower panels. (B) Cyclopamine induces Smo accumulation in the primary cilium but inhibits that of Gli2. (C) Smo agonist SAG1 induces accumulation of Gli2 and Smo in the cilium. (D–F) The amount of Gli2 in cilia (Gli2cil) and Gli2 in nuclei (Gli2nuc), Smo in cilia (Smocil) were quantified from complete Z-series of immunofluorescence images. (G) Gli-luciferase reporter activity was assayed in parallel. Error bars indicate SD. (H–J) Total extract, nuclear and cytoplasmic fractions were prepared from cells grown in parallel with A–F and analyzed by immunoblotting with anti-HA antibody. The Upper and Middle panels show full-length HA-Gli2 (HA-Gli2, arrow) and its repressor form (HA-Gli2R, arrowhead), respectively. Note the reciprocal accumulation of HA-Gli2 and HA-Gli2R in the absence or presence of ShhN in the nuclear extract. In the Lower panel, immunoblotting of the same extracts with a mixture of antibodies specific to lamin A/C and α-tubulin shows that only the nuclear extracts contain lamin A/C, and only the cytoplasmic extracts contain α-tubulin. Note that the total extract shows a strong α-tubulin band and also a faint lamin A/C band.