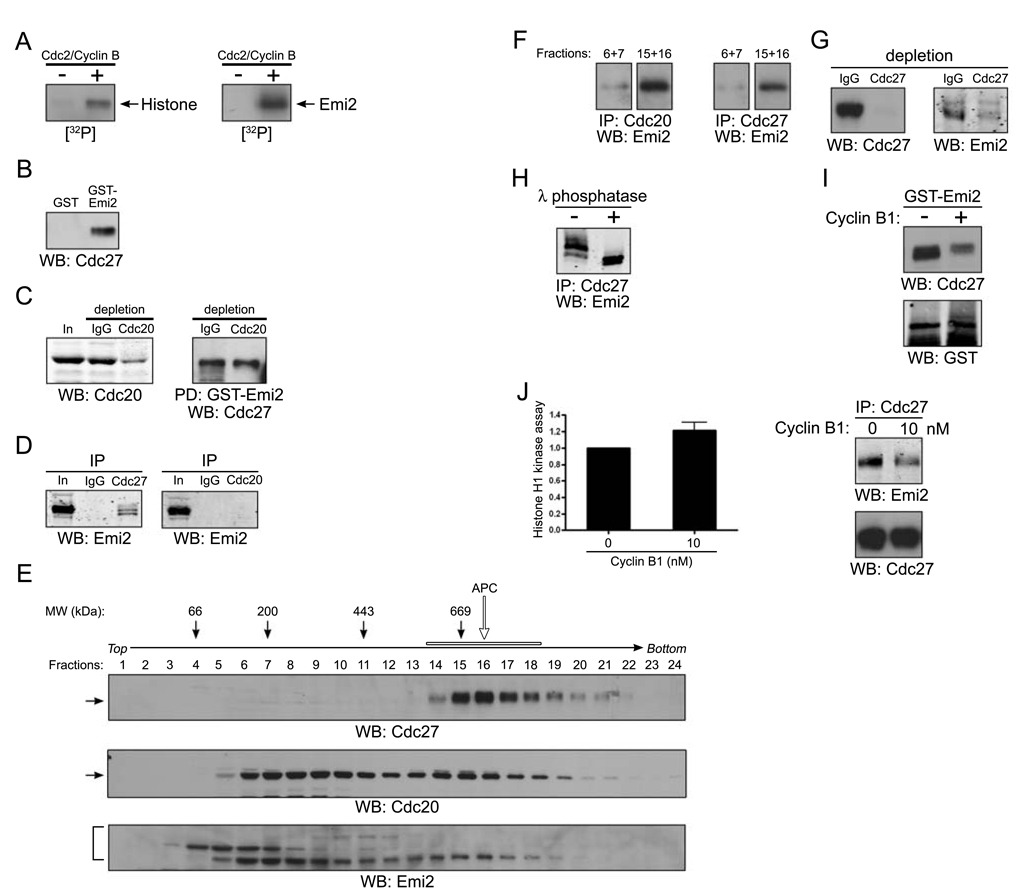
Figure 2. Cdc2/Cyclin B disrupts the association of Emi2 and APC/Cdc20.
(A) Histone H1 (left) or in vitro translated Emi2 (right) was mixed with [γ-32P] ATP in the presence or absence of recombinant Cdc2/Cyclin B kinase at room temperature for 10 min. The reactions were separated by SDS-PAGE and the phosphorylation of Histone H1 or Emi2 was monitored by autoradiography.
(B) GST or GST-Emi2 proteins bound to glutathione Sepharose beads were incubated in CSF extracts. After incubation, the protein beads were pelleted, washed, and immunoblotted for the core APC component, Cdc27.
(C) (Left): CSF extracts were depleted of Cdc20 by two consecutive incubations with Cdc20 antibody. The mock (IgG) and Cdc20 depleted extracts were resolved by SDS-PAGE and immunoblotted with anti-Cdc20 antibodies. (Right): GST-Emi2 linked to glutathione Sepharose was incubated in control or Cdc20 depleted extracts, retrieved, washed, and immunoblotted for Cdc27.
(D) Control IgG, Cdc27 (left) or Cdc20 (right) antibodies were immobilized on Protein A Sepharose beads and incubated in CSF extracts. The beads were retrieved, washed and immunoblotted for Emi2.
(E) CSF extracts was fractionated on a 5–30% sucrose gradient. Fractions were collected, analyzed by SDS-PAGE, and immunoblotted for Cdc27, Cdc20 and Emi2. The open arrow and bar indicate the APC complex.
(F) Cdc20 (left) or Cdc27 (right) antibodies were immobilized on Protein A Sepharose beads and incubated in sucrose gradient fractions 6 and 7 (pooled) or 15 and 16 (pooled). After incubation, the beads were retrieved by centrifugation, washed, and immunoblotted for Emi2.
(G) Cdc27 antibody was used to deplete CSF extract of the APC. IgG or Cdc27 depleted extracts were immunoblotted for Cdc27 (left) or Emi2 (right).
(H) Emi2 antibodies immobilized on Protein A Sepharose beads were incubated in CSF extracts. After incubation, the beads were retrieved, washed and incubated in the presence (+) or the absence (−) of λ phosphatase before being resolved by SDS-PAGE and immunoblotting for Emi2.
(I) GST-Emi2 linked to glutathione Sepharose was incubated in CSF extracts in the presence or absence of 80 nM recombinant Cyclin B1 proteins. After incubation, the protein beads were retrieved by centrifugation, washed, and immunoblotted for Cdc27 or GST.
(J) (Left): Cdc2/Cyclin B kinase activity was measured in CSF extracts in the presence or absence of exogenous recombinant Cyclin B1 (10 nM) using Histone H1 as an exogenous substrate. The phosphorylation of Histone H1 was quantitated and plotted. Error bars represent standard deviation of three measurements. (Right): Cdc27 antibodies were immobilized on Protein A Sepharose beads and incubated in CSF extracts in the presence or absence of Cyclin B1 (10 nM). The beads were retrieved, washed and immunoblotted for Emi2 or Cdc27.