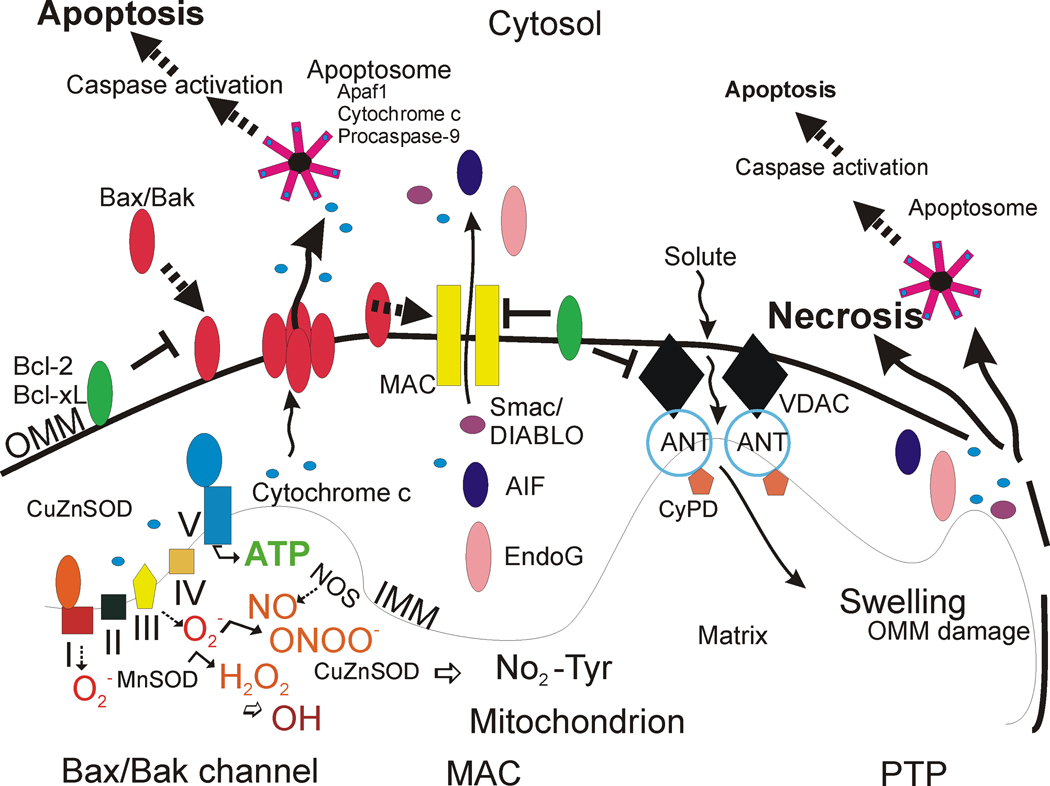
Figure 1. Mitochrondrial regulation of cell life and death in schematic representation.
The respiratory chain proteins (complex I–IV) establish an electrochemical gradient across the inner mitochondrial membrane (IMM) by extruding protons out of the matrix into the intermembrane space, thereby creating an energy gradient that drives the production on ATP by complex V (lower left). Superoxide (O2 •−) is produced as a by-product in the process of electron transport and is converted to hydrogen peroxide (H2O2) by MnSOD (or CuZnSOD). In pathological settings that can trigger cell aging and death, H2O2 can be converted to hydroxyl radical (OH), or hydroxyl-like intermediates, and mitochondrial nitric oxide synthase (NOS) can produce nitric oxide (NO) that can combine with O2 •− to form peroxynitrite (ONOO−). CuZnSOD can use ONOO− to catalyze the nitriation of mitochondrial protein tyrosine residues (bottom center) such as cyclophilin D (CyPD) and the adenine nucleotide translocator (ANT) [78]. Bcl-2 family members regulate apoptosis by modulating the release of cytochrome c from mitochondria into the cytosol. Two models can account for this process (the Bax/Bak channel model and the mitochondrial apoptosis-induced channel or MAC). In the Bax/Bak1 channel model (left), Bax (Bcl-2-associated X protein) is a pro-apoptotic protein found mostly in the cytosol in healthy mammalian cells but, after specific cell death inducing stumuli, Bax undergoes a conformation shift and translocates to the outer mitochondrial membrane (OMM) where it inserts [55,56]. Bak1 (Bcl-2-antagonist/killer 1) is a similar pro-apoptotic protein localized mostly to the mitochondrial outer membrane. Bax/Bak1 monomers physically interact and form oligomeric or heteromeric channels that are permeable to cytochrome c. The formation of these channels is blocked by Bcl-2 and Bcl-xL at multiple sites. BH3-only members (Bad, Bid, Noxa, Puma) are pro-apoptotic and can modulate the conformation of Bax/Bak1 to sensitize this channel, possibly by exposing its membrane insertion domain (not shown). The MAC could be a channel similar to the Bax/Bak1 channel, but it might also have additional components. Release cytochrome c participates in the formation of the apoptosome in the cytosol that drives the activation of caspase-3 in motor neurons (see Figure 2). Second mitochondria-derived activator of caspases (Smac)/ direct IAP-binding protein with low pI (DIABLO) are released into the cytosol to inactivate the anti-apoptotic actions of inhibitor of apoptosis proteins that inhibit caspases. The DNases AIF and EndoG are released and translocate to the nucleus to stimulate DNA fragmentation. Another model (right) for mitochondrial directed cell death involves the permeability transition pore (PTP). The PPT is a transmembrane channel formed by the interaction of the ANT and the voltage-dependent anion channel (VDAC) at contact sites between the IMM and the OMM [114–120]. CyPD, located in the matrix, can regulate the opening of the PTP by interacting with the ANT. Opening of the PTP induces matrix swelling and OMM rupture leading to release of cytochrome c and other apoptogenic proteins (AIF, EndoG). Certain Bcl-2 family members can modulate the activity of the PTP.