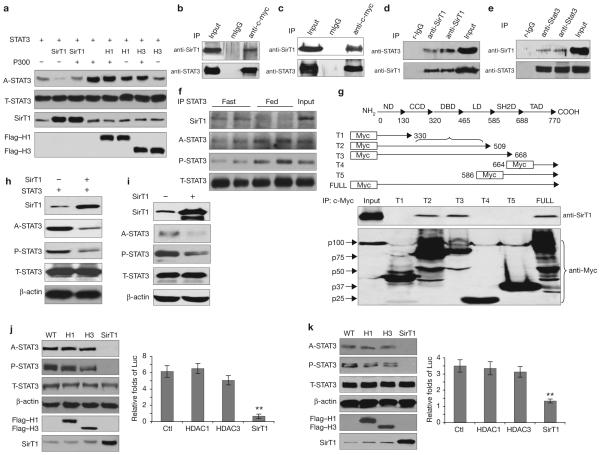
Figure 2.
STAT3 phosphorylation and transactivation were downregulated by SirT1. (a) SirT1 deacetylates STAT3 in cultured cells. The effect of p300, SirT1 or HDACs on STAT3 acetylation was measured in transfected HEK293T cells (H1, HDCA1; H3, HDCA3). (b-e) SirT1 and STAT3 form complexes in vitro and in vivo. HEK293T cells were transfected with SirT1 and STAT3, or STAT3 alone (b, c). The physical interactions between exogenous STAT3 and exogenous (b) or endogenous (c) SirT1 were detected. In HEK293T cells, endogenous SirT1 and endogenous STAT3 were co-precipitated (d). STAT3 and SirT1 were co-precipitated by a STAT3 antibody in the livers of wild-type male mice (n = 4, e). (f) The physical interaction between STAT3 and SirT1 was enhanced in fasting livers. (g) Except for full-length STAT3, SirT1 was only precipitated by truncated STAT3-T2 and -T3, suggesting that both the DNA binding and the linker domains of STAT3 are involved in the interaction of STAT3 and SirT1. ND, N-terminal domain; CCD, coil-coil domain; DBD, DNA binding domain; LD, linker domain; SH2D, SH2 domain and TAD, transactivation domain. (h, i) SirT1-mediated deacetylation of STAT3 affects Y705-STAT3 phosphorylation. HEK293T cells were transfected with SirT1 and STAT3 (0.25 μg per well of each, h), or SirT1 alone (i), in 12-well-plates. (i) Each well was loaded with 100 μg of total protein to visually present the signals of endogenous A-STAT3 and P-STAT3. (j, k) The SirT1-mediated deacetylation of STAT3 affected STAT3 function (WT, wild-type). A2780 cells were treated with IL-6 (40 ng ml-1) for 12 h. A relatively low level of the SirT1, HDAC1 and HDAC3 plasmids (0.05 μg per well) were either transfected with STAT3 (0.1 μg per well) or untreated (control). The effects on either exogenous (20 μg per well, j) or endogenous (60 μg per well, k) STAT3 acetylation and phosphorylation were determined. STAT3 transactivation activities were detected by a STAT3 specific luciferase reporter (Luc). Data are mean ± s.e.m. of three repeated experiments, ** P < 0.01 in J and k.