Abstract
Aims
The study aimed to estimate incidence rates of mild cognitive impairment and related disorders, and conversion to dementia.
Methods
The data are drawn from the PATH Through Life Study. Baseline assessment in 2001–2002 included 2,551 participants 60–64 years old with 2,222 participating in a 4-year follow-up. Those screened positive with a cognitive assessment received clinical assessment for diagnoses of mild cognitive disorders (MCD) or dementia using established clinical criteria. Prevalence and incidence rates for the cohort were estimated with predictive regression models.
Results
Annual incidence of dementia was 0.25%. Prevalence of mild cognitive impairment was 4.2%, age-associated memory impairment was 2.4%, age-associated cognitive decline was 7.6%, mild neurocognitive disorders occurred in 12.9% and other cognitive disorders in 7.3%. Prevalence of any diagnosis of any MCD (Any-MCD) was 29.5% and the annual incidence rate for Any-MCD was 5.7%. Agreement for specific diagnoses between waves 1 and 2 was fair to poor (0–47.0%), but agreement for Any-MCD over 4 years was 89.0%.
Conclusion
MCD diagnoses do not predict dementia at a 4-year follow-up in young-old adults. Prevalence rates for MCD vary greatly depending on the criteria and time of assessment.
Key Words: Cognition disorders, Cognitive decline, Cognitive tests, Early diagnosis, Epidemiology, Longitudinal assessment, Mild cognitive impairment
Introduction
It is widely recognised that very early identification of cognitive decline is important for targeting with treatments and interventions that may prevent or delay dementia [1, 2]. Several sets of criteria have been proposed to describe cognitive impairment in late life that is not severe enough to meet criteria for dementia [3], with particular research focus on mild cognitive impairment (MCI) [4] as defined by the Mayo Clinic Group [5] and particular sub-types of MCI [6]. Prevalence rates of mild cognitive disorders (MCD) in community samples of older adults are notably variable [3] and have ranged from 3 to 36% [7]. Likewise there is wide variation in the reported conversion rates from MCD to dementia, ranging from 1 to 36% per year [3]. Reasons proposed for this variability include variation in the sampling frame, diagnostic criteria, underlying neuropathology and sample age [8]. In a review of 19 longitudinal studies published between 1991 and 2001, it was concluded that the major source of variability in the conversion rate is the study setting with the memory clinic attendees having higher conversion rates compared to epidemiological samples [9]. Amnestic MCI diagnosis with its emphasis on memory impairment alone has been highly criticised due its poor predictive validity [10]. As a result, MCI sub-types have been included in the diagnostic framework of subjects presenting with cognitive complaints [11]. It is also important to note that MCI is clearly a heterogeneous state in the community setting, where more than a third of subjects revert to normal [12,13,14]. In the past, results on the predictive validity of MCI diagnoses in population-based samples have been disappointing [10, 15], yet such studies are required to obtain true estimates of cognitive disorders in the population. These are required for public health planning, costing and interventions. Although sensitive neuropsychological markers such as tests of episodic memory [16] increase the accuracy of prediction, there is a growing consensus that the best model of prediction should use a multivariate model where researchers combine clinical judgement with neuroimaging and biomarkers [4]. This may be possible in clinical settings, but the implementation of this at the primary care level would be difficult. We have reported previously in the same sample (wave 1) that even with the use of structural MRIs, there was no difference between MCI subjects and normal controls in medial temporal lobe atrophy, general cortical atrophy and white-matter hyperintensities [17]. Therefore, it is crucial to develop valid clinical criteria with good stability and predictability that can be effectively used in epidemiological and primary-care settings. Recent refinements to criteria for MCI have yielded more valid diagnoses [11, 18, 19], but relatively few prevalence or incidence data are available on MCD diagnoses in late middle age.
Schonknecht et al. [20] followed a sample of 500 adults aged in their early 60s (mean age 62.4, SD 2.4) for 4 years and found that the prevalence of age-associated cognitive decline (AACD) was 23.6%, with an additional 7.8% of participants reaching criteria for MCD, but no cases identified at baseline converted to dementia during the follow-up period. This study suggested that conversion rates to dementia were very low in the young old with MCD, consistent with population prevalence rates [21]. In adults aged 65 and older, diagnoses of MCI have been shown to be relatively unstable [14]. Follow-up of young-old cohorts therefore need to focus on the extent to which participants retain diagnoses over time, and the length of time individuals may retain a diagnosis before converting to dementia in long-term follow-up studies [7].
The aims of the present study were to estimate the prevalence and incidence of MCD in a 4-year follow-up of a population-based sample of adults aged in their early 60s at baseline, to determine the rate of conversion to dementia in this sample and to evaluate the stability of MCD diagnoses.
Methods
Participants were sampled randomly from the electoral rolls for Canberra, A.C.T., and Queanbeyan, N.S.W., Australia, as part of the PATH Through Life Project which involves approximately 2,500 persons in each of three age groups, 20–24, 40–44 and 60–64 years [22]. They were asked to complete a questionnaire under the supervision of a professional interviewer. Some basic physical tests were also carried out (e.g. blood pressure, grip strength, visual acuity and lung function) and a cheek swab was taken from which DNA could be extracted. Written, informed consent was obtained, and the relevant institutional ethics committees and the Australian National University Ethics Committee approved the study. Results presented here concern the first- and second-wave interview with 60- to 64-year-olds, conducted in 2001–2002 and 2005–2006, respectively. Of 4,831 people contacted, 2,551 were interviewed in wave 1 (58.3% of those found and in age range) and 2,222 were interviewed in wave 2. The design of the PATH Through Life Study and the methodology for clinical diagnoses has been described elsewhere [8, 23, 24].
Screening
At each wave, the same, predetermined cut-off on a cognitive screening battery was used to screen participants in a sub-study on MCD and dementia. Participants from the full cohort were selected for clinical assessment if they had any of the following: (a) a Mini-Mental State Examination (MMSE) [25] score ≤25; (b) a score below the 5th percentile score from wave 1 on immediate or delayed recall of the California Verbal Learning Test [26] (immediate or delayed score of <4 and <2, respectively), or (c) a score below the 5th percentile score for wave 1 on two or more of the following tests: Symbol-Digit Modalities Test(SDMT; <33) [27] or Purdue Pegboard with both hands [28] (Purdue; wave 1: <8; wave 2 <7) or simple reaction time [29] (SRT; third set of 20 trials; wave 1: >310 ms; wave 2: >378 ms).
Clinical Assessment
The clinical assessment involved a Structured Clinical Assessment for Dementia (available from the authors) by one of two physicians, a neuropsychological assessment and the Clinical Dementia Rating (CDR) scale [30]. Information was also gathered on medical history related to cognitive function, duration of symptoms, medical history from medical practitioners and family, current treatment and psychiatric history. Depression was assessed using the self-administered Patient Health Questionnaire (PHQ) from the Primary Care Evaluation of Mental Disorders PRIME-MD [31]. Informant interviews were conducted where possible. Participants receiving any clinical diagnosis were referred to their family doctor for laboratory investigations. The research protocol included MRI scans for all consenting participants. The neuropsychological assessment included frontal executive function (trails A and B [32], verbal fluency [33] and clock drawing [34]), language (short form of the Boston Naming Test [35]), constructional praxis from the Consortium to Establish a Registry for Alzheimer's Disease [36], memory (Rey Auditory Verbal Learning Test with verbal recall and recognition [37]) recall of constructional praxis for non-verbal memory and agnosia [38].
Clinicians used clinical checklists, data from the neuropsychological assessment, neuropsychiatric history and medical history in formulating consensus diagnoses. Criteria for the following diagnoses were applied: MCI [39], age-associated memory impairment (AAMI) [40], AACD [41], mild neurocognitive disorder (MND) [42], impairment on the CDR [30] and other cognitive disorders (OCD) [42]. DSM-IV criteria were used to assess dementia and delirium [42]. The diagnostic criteria for the above diagnoses has been published by our group elsewhere [8]. Although this study focuses on MCD (MCI, AAMI, AACD, MND, impaired CDR and OCD), diagnoses of dementia, delirium and amnestic disorders are reported. Importantly for this study, clinicians were blind to the diagnosis or lack of diagnosis obtained at wave 1.
Statistical Analysis
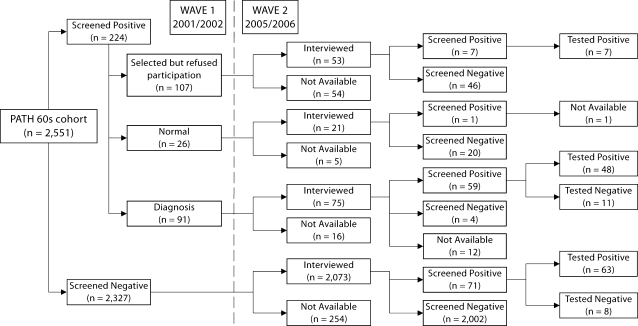
To establish predicted prevalence and incidence rates, predictive regression models were built based on the relationship between the screening measures and the clinical data for the sub-sample for whom diagnostic data were available (n = 137), as described in figure 1. It was necessary to compute prevalence among participants who screened positive but who did not undertake the clinical assessment, and to estimate the false positives in the sample that screened negative. Using the clinical diagnoses as the gold standard, logistic regressions with age, sex and screening measures as predictor variables were built. For each diagnostic criterion, a predictive score defined as the probability of a positive diagnosis was derived, and a cut-off point was chosen so that the number of predicted diagnoses was the same as the number of observed diagnoses under the criterion in the sub-sample. This cut-off point was then applied to the predictive score of: (a) those that screened positive but did not undertake the clinical assessment and (b) those who screened negative. The final prevalence estimate was a sum of those receiving clinical diagnoses, those estimated to receive a diagnosis among the group that screened positive but did not receive a diagnosis, and those estimated to have been falsely screened as negative. Note that this prevalence estimate assumes that the relationship between clinical diagnosis and screening measures among those who screened positive but did not undertake the clinical assessment and those who screened negative is essentially the same as in the sub-sample for whom diagnostic data were available.
Fig. 1.
Flow chart showing sample participation and diagnosis at waves 1 and 2.
Only MMSE, and immediate and delayed recall were significant predictors of obtaining any MCD (Any-MCD), but the classification rate did not change when delayed recall was removed, so the final model used to estimate the probability of diagnosis included a constant, MMSE and immediate recall. These variables were retained in the logistic regression analyses for estimating the weights used to calculated prevalence estimates for all disorders. Confidence intervals and standard errors for the prevalence estimate were estimated using bootstrap techniques. One thousand bootstrap samples were generated. Each bootstrap sample consisted of the same number of subjects (n = 137) who were sampled with replacement from the list of subjects for whom diagnostic data were available. For each bootstrap sample, the logistic regression linking the diagnostic data and the screening tests was fitted. By performing this calculation, we had a list of 1,000 bootstrap prevalence estimates from the 1,000 bootstrap samples. The population prevalence estimate was then calculated as the average of prevalence estimates across bootstrap samples. The 95% confidence interval was calculated by taking the 2.5th and 97.5th percentile of the bootstrap prevalence estimates.
Comparisons of cognitive measures and depression scores between diagnostic groups over the two waves were conducted using standardised residual change scores. Participants were classified into four groups (group 1: no diagnosis at wave 1 or wave 2; group 2: diagnosis at wave 1 but not at wave 2; group 3: no diagnosis at wave 1 but diagnosis at wave 2, and group 4: diagnosis at both waves). Residual change scores were computed as the difference between the predicted wave 2 score and the observed wave 2 score. These scores were standardised to have a mean of zero and a standard deviation of one. Groups were compared on these scores using one-way analysis of variance (ANOVA), with Dunnett T3 post hoc tests. All analyses were conducted in SPSS 15.00 and STATA version 9.0.
Results
Description of the Sample
Figure 1 is a flow chart showing the study sample. Those who screened positive but declined clinical assessment at wave 2 were compared with those who screened positive and were assessed at wave 2. They did not differ in age, sex, English-speaking background or medical conditions, including hypertension, head injury, stroke, diabetes, asthma, cataract, arthritis, cancer, thyroid or heart disease. Those participants who refused assessment had fewer years of education [11.16 vs. 12.12, t(212) = −2.16, p = 0.03] and were more likely to smoke [28.57 vs. 11.11%, χ2 (1, 1) = 10.978, p < 0.001].
At wave 2, there were 43 cases of MCI, 32 cases of AAMI, 60 cases of AACD, 21 cases of MND, 6 cases of OCD, 116 participants with impaired CDR and 2 cases of dementia. In total, there were 117 participants with diagnoses of Any-MCD.
Wave 2 Prevalence and Incidence of Diagnoses
Regression-based prevalence estimates and their estimated intervals derived from resampling (shown in parentheses) at wave 2 were 2.44% (2.31–3.37%) for AAMI, 4.20% (3.80–5.25%) for MCI, 7.57% (7.33–8.11%) for AACD, 12.87% (1.45–80.47%) for MND, 7.29% (0.04–46.60%) for OCD and 21.00% (19.33–30.23%) for impaired CDR. The prevalence of Any-MCD was 29.51% (21.87–36.89%). The width of the confidence intervals varies for different criteria and depends on how well defined the relationships are between the screening measures and the criteria in the sub-sample. Criteria that have well-defined relationships with the screening measures, indicated by small standard errors for their regression coefficients, will have tight confidence intervals.
Calculation of stability of clinical diagnoses over 4 years was based on participants receiving clinical assessments at both waves. Of those diagnosed with MCI at wave 1, 28.57% retained this diagnosis at wave 2, stability of AAMI was 1.00%, AACD was 46.67%, MND was 33.00%, OCD was 25.00% and impaired CDR was 80.00%. Stability of Any-MCD was 88.88%.
Given the low stability and the large interval estimates for some of the specific MCD, incidence data are only reported for receiving a diagnosis of Any-MCD. To estimate the incidence of diagnoses of Any-MCD at wave 2, participants with diagnoses or estimated diagnoses at wave 1 (from the group screened positive but who did not have clinical assessment at wave 1) were excluded. There were 496/2,158 incident cases of Any-MCD, yielding an incidence rate of 57.46 per 1,000 per annum or 5.70% (4.23–7.17%).
Characteristics of Clinical Groups at Both Waves
Table 1 shows the demographic characteristics of participants according to diagnosis group. One-way ANOVA showed overall effects between groups on years of education [F (3, 2419) = 17.435, p ≤ 0.001], but the four groups did not differ in age [F (3, 2546) = 0.717, p = 0.542]. Scheffé analyses revealed that participants who were classified as ‘no diagnosis’ in either wave (group 1), reported higher levels of education than the three diagnosis groups (p < 0.001).
Table 1.
Demographic characteristics [means (SD) or number of cases] for participants according to clinical diagnosis status at wave 1 and wave 2
| Variable | Group 1 normal to normal (n = 2,389) | Group 2 diagnosis to normal (n = 43) | Group 3 normal to diagnosis (n = 71) | Group 4 diagnosis to diagnosis (n = 48) | F/χ2a | p |
|---|---|---|---|---|---|---|
| Age (wave 1), years | 62.51 (1.50) | 62.81 (1.47) | 62.49 (1.61) | 62.38 (1.51) | 0.717 | 0.542 |
| Male/female | 1,214/1,175 | 28/15 | 40/31 | 35/13 | 13.099 | 0.004 |
| English, yes/no | 2,109/277 | 28/15 | 57/14 | 32/16 | 43.389 | 0.000 |
| Years of education | 13.98 (2.80) | 11.71 (3.04) | 12.66 (2.83) | 12.12 (3.43) | 17.435 | 0.000 |
F test was measured using one-way ANOVA (post hoc: Scheffé); χ2 was assessed using a Kruskal-Wallis test (post hoc: Mann-Whitney).
Kruskal-Wallis tests showed overall effects between groups on the categorical variables gender [χ2 (3) = 13.099; p < 0.01] and English as a first language [χ2 (3) = 43.389; p < 0.001]. Post hoc analyses showed a greater proportion of males in group 4 (p < 0.01) and a greater proportion of participants reporting English as their first language in group 1 (p < 0.01).
Table 2 shows the raw scores (means ± SD) on the screening tests for the four diagnostic groups. Group 1 reported higher scores (p < 0.01) than the three other groups on all cognitive screening variables except SRT at wave 1 [group 1 faster than groups 2 (p < 0.01) and 4 (p < 0.05)] and Purdue at wave 2 [group 1 higher than groups 3 (p < 0.05) and 4 (p < 0.01) only]. Group 1 scored lower than group 2 (p < 0.01) on the PHQ at wave 1. Group 3 reported higher scores (p < 0.001) than groups 2 and 4 on MMSE, and immediate and delayed recall. Finally, group 4 reported higher scores on the PHQ at wave 2 compared to groups 1 (p < 0.001) and 2 (p < 0.05).
Table 2.
Screening measures [means (SD)] for participants according to clinical diagnosis status at wave 1 (W1) and wave 2 (W2)
| Variable | Group 1 normal to normal (n = 2,386) | Group 2 diagnosis to normal (n = 43) | Group 3 normal to diagnosis (n = 71) | Group 4 diagnosis to diagnosis (n = 48) | Fa (p) |
|---|---|---|---|---|---|
| MMSE W1 | 29.21 (1.31) | 26.64 (2.77) | 28.36 (2.19) | 26.85 (2.61) | 94.656 (0.000) |
| MMSE W2 | 29.29 (1.05) | 28.04 (1.81) | 27.45 (2.45) | 27.20 (2.55) | |
| Recall | |||||
| Immediate W1 | 7.28 (2.20) | 3.72 (1.94) | 5.61 (1.76) | 3.71 (1.82) | 89.744 (0.000) |
| Immediate W2 | 7.11 (2.13) | 5.65 (1.67) | 3.79 (1.46) | 4.46 (1.66) | |
| Delayed W1 | 6.33 (2.42) | 2.84 (1.86) | 4.62 (1.80) | 2.29 (1.91) | 83.210 (0.000) |
| Delayed W2 | 6.29 (2.30) | 4.23 (1.90) | 2.99 (1.82) | 3.52 (1.83) | |
| SDMT W1 | 50.23 (9.40) | 38.90 (11.53) | 43.24 (11.61) | 39.94 (11.01) | 47.637 (0.000) |
| SDMT W2 | 49.95 (8.96) | 43.12 (8.95) | 40.61 (11.48) | 38.83 (10.90) | |
| Purdue W1 | 10.47 (1.71) | 9.27 (2.30) | 9.81 (1.81) | 9.40 (1.94) | 14.904 (0.000) |
| Purdue W2 | 10.49 (1.79) | 9.50 (2.44) | 9.40 (2.24) | 8.96 (1.96) | |
| SRT W1 | 0.26 (0.06) | 0.29 (0.10) | 0.27 (0.08) | 0.28 (0.07) | 8.895 (0.000) |
| SRT W2 | 0.27 (0.07) | 0.34 (0.14) | 0.30 (0.08) | 0.28 (0.08) | |
| PHQ W1 | 2.29 (3.00) | 2.92 (3.92) | 2.82 (3.67) | 3.13 (4.94) | 5.987 (0.000) |
| PHQ W2 | 2.41 (3.12) | 2.82 (3.67) | 3.83 (4.94) | 4.70 (4.95) |
F test is for the one-way ANOVA on residualized change scores, d.f. (3, 2544).
Additional analyses tested for differences between groups in the amount of change in the screening tests between waves. Overall differences between diagnostic groups in residual change scores are reported in the final column of table 2. A significant Levene statistic (p < 0.05) indicated equality of variances was not present between groups on any test. Examination of both the Welch and Brown-Forsythe robust F-statistics revealed significant differences between diagnostic groups on all outcome variables except the PHQ (Welch = 2.693, p = 0.051; Brown-Forsythe F = 3.11; p = 0.029). Non-parametric analysis of the data using the Kruskal-Wallis test confirmed these findings, with differences in all of the outcome variables except the PHQ [χ2 (3) = 4.962, p = 0.175].
Group 1 reported significantly less change than the other three groups in the MMSE, immediate and delayed recall, SDMT and Purdue (p < 0.001), but not on the SRT. Group 3 reported significantly less change than group 2 and group 4 on the MMSE, immediate and delayed recall (p < 0.001), but not on the SDMT, Purdue or SRT. Groups 2 and 4 reported comparable change in all cognitive measures. Analyses showed that residual change scores for immediate or delayed recall did not differ for groups 2 and 4, with both groups showing small practice effects resulting in an average of about 4 words retrieved for delayed recall at wave 2.
Discussion
The present study reports valuable information on the prevalence, incidence and stability of diagnoses of MCD in a population-based sample of adults aged in their 60s. Unlike older samples where conversion rates of MCI to dementia are reported to be approximately 10–30% [43,44,45], there was no conversion to dementia in this young sample. This is consistent with other research on MCI in this age group [20]. Our estimates of an annual incidence of 5.7% (4.23–7.17%) for any cognitive disorder and our estimate of 29.51% (21.87–36.89%) of the sample experiencing MCD at follow-up are similar to others reported for this age group [20]. However, they are higher than population-based estimates of cognitive impairment not reaching the threshold for dementia in the United States [46]. Results from the Ageing, Demographics and Memory Study found the mean MMSE for participants with cognitive impairment without dementia was 24.75, which is substantially lower than the mean MMSE scores of the Any-MCD group in this study, but the mean age in the above-mentioned study was much older and only 56% of that sample completed the initial cognitive assessment. These estimates in the current study appear high for such a young age group. This is partly due to the normative nature of the diagnostic criteria. In the normal distribution, 16% of the sample will score one or more standard deviations below the mean on any measure by definition.
Our results showed poor to fair stability for individual diagnoses, with only 29% of MCI participants and 1% of AAMI participants rediagnosed after 4 years. These results are consistent with other population-based studies showing that AACD is more stable than MCI [10], and that more participants are classified with this diagnosis [10, 47]. A likely explanation for the greater stability of the AACD diagnosis is that unlike the original MCI diagnosis used in this study, the objective and subjective (informant or self-assessed) cognitive deficit is not restricted to memory, but may include deficits in one of several cognitive domains. The extremely low stability of the AAMI diagnosis calls into question its validity and suggests that some of the individual criteria for this diagnosis are arbitrary.
An important finding from the present study is that individuals who received an Any-MCD diagnosis (MCI or AAMI or AACD or MND or OCD or MCD) had an 89% chance of receiving a diagnosis of a cognitive disorder after 4 years. It is possible that the individuals receiving such diagnoses have very low pre-morbid cognitive ability, and hence, relative to the population, will always receive scores that are in the impaired range. A detailed history of cognitive change is therefore required to distinguish those with pathological cognitive decline from those with low pre-morbid cognitive ability in this age group.
These results clearly show that in this very young sample, clinical assessments of cognitive disorders are stable and reliable at a general level, but the specificity of diagnoses according to the range of clinical criteria is not. Recent developments in refining the criteria for diagnosis of MCI have acknowledged the complexity of operationalising the definition [11] in much older samples than that used in the present study, but it remains unclear whether there is any benefit in refining definitions of these disorders for this age group.
Although an MMSE score of 25 was used as a screening threshold to select participants for clinical assessment, the average MMSE scores of those receiving diagnoses at wave 1 were over 26. This suggests that a higher threshold on the MMSE is required to screen for mild cognitive disorders in this relatively young sample. Our screening measures included a range of tests, but analyses at both waves showed that only the MMSE and word recall task were significant in predicting who achieved a diagnosis in this age group. Moreover, the mean score on the MMSE obtained by those receiving clinical diagnoses was relatively high. Other cognitive measures are important for characterising the nature of cognitive decline and may be predictive in older samples. Extending the recall component of the MMSE would provide a simple and short method of screening for MCD in this age group.
One possible reason for participants being misclassified with Any-MCD may be that impaired cognition is a consequence of depression and hence remission of depressive symptoms may result in remission of cognitive impairment. Depression and depressive symptoms have been shown to be more prevalent in those with Any-MCD [48]. We investigated this possibility as an explanation for the low stability of diagnoses, but did not find any difference between groups in the residual change scores of the PHQ.
Our study was limited by the response rate to the clinical assessment at wave 1, the narrow age range and the small numbers of participants in each diagnostic group. At wave 2, 50% of those who declined the clinical assessment at wave 1 were lost to follow-up. This is consistent with previous research showing that poor cognitive performance is associated with study dropout and non-response [49]. The much improved response rate at wave 2 may have been due the loss of this earlier non-responding group from the total sample, and increased commitment to the study from those who persisted. Another limitation of the study was the two-stage sampling design, and the lack of clinical investigation of potential screen negatives from the larger sample. However, in such a large cohort, conducting full clinical assessments on all participants is resource intensive and may increase participant dropout.
The small numbers of specific diagnoses also led to large interval estimates that are unreliable. The study was also limited by the long follow-up interval, with more reliable estimates likely to be achieved with more occasions of measurement. Inclusion of participants for whom English was not their first language may have also inflated the observed numbers of Any-MCD. It is unlikely that education levels affected the sensitivity of the MMSE as research has shown that education differences account for a very small proportion of variance in this measure [50]. The evaluation of cognitive change within diagnostic groups may be confounded by practice effects, making it difficult to identify true improvement in the diagnosis-to-normal group. Multiple occasions of measurement would allow for statistical modelling and separation of practice effects from true change [51].
Study strengths are that it drew from a larger population-based cohort of over 2,500 participants and presents one of the largest prospective population-based studies of MCD adults aged in their 60s. It is also one of the few studies to report data on the stability of diagnoses in this age group. Follow-up of this sample will determine what proportion of participants classified as Any-MCD convert to dementia. This will allow for better estimates of duration of detectable MCI prior to dementia, and the evaluation of the significance of clinical diagnoses made in late middle age. Such information is essential for indicating early intervention or prevention of dementia through lifestyle changes or medication.
Acknowledgements
We thank the study participants, PATH Interviewers, Patricia Jacomb, Karen Maxwell and Bryan Rodgers. The PATH Through Life Study is funded by National Health and Medical Research Council grants (229936 and 179839). K.J.A. is supported by NHMRC fellowship 366756; H.C. by NHMRC fellowship 366781; A.F.J. by NHMRC Fellowship 40001, and N.C. in part by Alzheimer's Australia.
References
- 1.Panza F, D'Introno A, Colacicco AM, Capurso C, Parigi AD, Capurso SA, Caselli RJ, Pilotto A, Scafato E, Capurso A, Solfrizzi V. Cognitive frailty: predementia syndrome and vascular risk factors. Neurobiol Aging. 2006;27:933–940. doi: 10.1016/j.neurobiolaging.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Levey A, Lah J, Goldstein F, Steenland K, Bliwise D. Mild cognitive impairment: an opportunity to identify patients at high risk for progression to Alzheimer's disease. Clin Ther. 2006;28:991–1001. doi: 10.1016/j.clinthera.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Tuokko HA, McDowell I. An overview of mild cognitive impairment. In: Tuokko HA, Hultsch DF, editors. Mild Cognitive Impairment: International Perspectives. New York: Taylor & Francis; 2006. pp. 3–28. [Google Scholar]
- 4.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 7.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Results of the Leipzig Longitudinal Study of the Aged (LEILA75+). Br J Psychiatry. 2003;182:449–454. [PubMed] [Google Scholar]
- 8.Kumar R, Dear KB, Christensen H, Ilschner S, Jorm AF, Meslin C, Rosenman SJ, Sachdev PS. Prevalence of mild cognitive impairment in 60- to 64-year-old community-dwelling individuals: the Personality and Total Health through Life 60+ Study. Dement Geriatr Cogn Disord. 2005;19:67–74. doi: 10.1159/000082351. [DOI] [PubMed] [Google Scholar]
- 9.Bruscoli M, Lovestone S. Is MCI really just early dementia?. A systematic review of conversion studies. Int Psychogeriatr. 2004;16:129–140. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Artero S, Petersen R, Touchon J, Ritchie K. Revised criteria for mild cognitive impairment: validation within a longitudinal population study. Dement Geriatr Cogn Disord. 2006;22:465–470. doi: 10.1159/000096287. [DOI] [PubMed] [Google Scholar]
- 12.Palmer K, Fratiglioni L, Winblad B. What is mild cognitive impairment?. Variations in definitions and evolution of nondemented persons with cognitive impairment. Acta Neurol Scand Suppl. 2003;179:14–20. doi: 10.1034/j.1600-0404.107.s179.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Ganguli M. Mild cognitive impairment and the 7 uses of epidemiology. Alzheimer Dis Assoc Disord. 2006;20:S52–S57. doi: 10.1097/00002093-200607001-00007. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 15.Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: prevalence and predictive validity according to current approaches. Acta Neurol Scand. 2003;108:71–81. doi: 10.1034/j.1600-0404.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 16.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumar R, Parslow RA, Jorm AF, Rosenman SJ, Maller J, Meslin C, Anstey KJ, Christensen H, Sachdev PS. Clinical and neuroimaging correlates of mild cognitive impairment in a middle-aged community sample: the personality and total health through life 60+ study. Dement Geriatr Cogn Disord. 2006;21:44–50. doi: 10.1159/000089251. [DOI] [PubMed] [Google Scholar]
- 18.Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: a 3-year follow-up study. J Am Geriatr Soc. 2006;54:580–586. doi: 10.1111/j.1532-5415.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 19.Lopez OL, Becker JT, Jagust WJ, Fitzpatrick A, Carlson MC, DeKosky ST, Breitner J, Lyketsos CG, Jones B, Kawas C, Kuller LH. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schonknecht P, Pantel J, Kruse A, Schroder J. Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. Am J Psychiatry. 2005;162:2071–2077. doi: 10.1176/appi.ajp.162.11.2071. [DOI] [PubMed] [Google Scholar]
- 21.Jorm AF, Windsor TD, Dear KBG, Anstey KJ, Christensen H, Rodgers B. Age group differences in psychological distress: the role of psychosocial risk factors that vary with age. Psychol Med. 2005;35:1253–1263. doi: 10.1017/S0033291705004976. [DOI] [PubMed] [Google Scholar]
- 22.Jorm AF, Anstey KJ, Christensen H, Rodgers B. Gender differences in cognitive abilities: the mediating role of health state and health habits. Intelligence. 2004;32:7–23. [Google Scholar]
- 23.Anstey KJ, Mack HA, Christensen H, Li SC, Reglade-Meslin C, Maller J, Kumar R, Dear K, Easteal S, Sachdev P. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45:1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Christensen H, Dear KB, Anstey KJ, Parslow RA, Sachdev P, Jorm AF. Within-occasion intraindividual variability and preclinical diagnostic status: is intraindividual variability an indicator of mild cognitive impairment? Neuropsychology. 2005;19:309–317. doi: 10.1037/0894-4105.19.3.309. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio: Psychological Corporation/Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 27.Smith A. Symbol Digit Modalities Test (SDMT) Manual. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 28.Tiffin J. Examiners Manual. Rosemont: London House; 1968. Purdue Pegboard. [Google Scholar]
- 29.Anstey KJ, Dear K, Christensen H, Jorm AF. Biomarkers, health, lifestyle and demographic variables as correlates of reaction time performance in early, middle and late adulthood. Q J Exp Psychol. 2005;58A:5–21. doi: 10.1080/02724980443000232. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 32.Reitan RM. Trail making test results for normal and brain-damaged children. Percept Mot Skills. 1971;33:575–581. doi: 10.2466/pms.1971.33.2.575. [DOI] [PubMed] [Google Scholar]
- 33.Lezak MD. Neuropsychological Assessment. Oxford: Oxford University Press; 1995. [Google Scholar]
- 34.Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, Grafman JH. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37:725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 35.Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer's disease. J Gerontol. 1992;47:P154–P158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- 36.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 37.Rey A. Psychological examination of traumatic encephalopathy. Arch Psychol. 1941;28:286–340. [Google Scholar]
- 38.Kertesz A. Western Aphasia Battery. San Antonio: Psychological Corporation; 1983. [Google Scholar]
- 39.Jack CR, Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J. 1962;86:257–260. [PMC free article] [PubMed] [Google Scholar]
- 41.Crook T, Bahar H, Sudilovsky A. Age-associated memory impairment: diagnostic criteria and treatment strategies. Int J Neurol. 1987;21–22:73–82. [PubMed] [Google Scholar]
- 42.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington: American Psychiatric Association; 1994. [Google Scholar]
- 43.Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 44.Maioli F, Coveri M, Pagni P, Chiandetti C, Marchetti C, Ciarrocchi R, Ruggero C, Nativio V, Onesti A, D'Anastasio C, Pedone V. Conversion of mild cognitive impairment to dementia in elderly subjects: a preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr. 2007;44(suppl 1):233–241. doi: 10.1016/j.archger.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 45.Rossini PM, Del Percio C, Pasqualetti P, Cassetta E, Binetti G, Dal Forno G, Ferreri F, Frisoni G, Chiovenda P, Miniussi C, Parisi L, Tombini M, Vecchio F, Babiloni C. Conversion from mild cognitive impairment to Alzheimer's disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143:793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, McArdle JJ, Willis RJ, Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanninen T, Koivisto K, Reinikainen KJ, Helkala EL, Soininen H, Mykkanen L, Laakso M, Riekkinen PJ. Prevalence of ageing-associated cognitive decline in an elderly population. Age Ageing. 1996;25:201–205. doi: 10.1093/ageing/25.3.201. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Jorm AF, Parslow RA, Sachdev PS. Depression in mild cognitive impairment in a community sample of individuals 60–64 years old. Int Psychogeriatr. 2006;18:471–480. doi: 10.1017/S1041610205003005. [DOI] [PubMed] [Google Scholar]
- 49.Anstey KJ, Luszcz MA. Selective non-response to clinical assessment in the longitudinal study of aging: implications for estimating population levels of cognitive function and dementia. Int J Geriatr Psychiatry. 2002;17:704–709. doi: 10.1002/gps.651. [DOI] [PubMed] [Google Scholar]
- 50.Jones RN, Gallo JJ. Education bias in the mini-mental state examination. Int Psychogeriatr. 2001;13:299–310. doi: 10.1017/s1041610201007694. [DOI] [PubMed] [Google Scholar]
- 51.Ferrer E, Salthouse TA, Stewart WF, Schwartz BS. Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychol Aging. 2004;19:243–259. doi: 10.1037/0882-7974.19.2.243. [DOI] [PubMed] [Google Scholar]