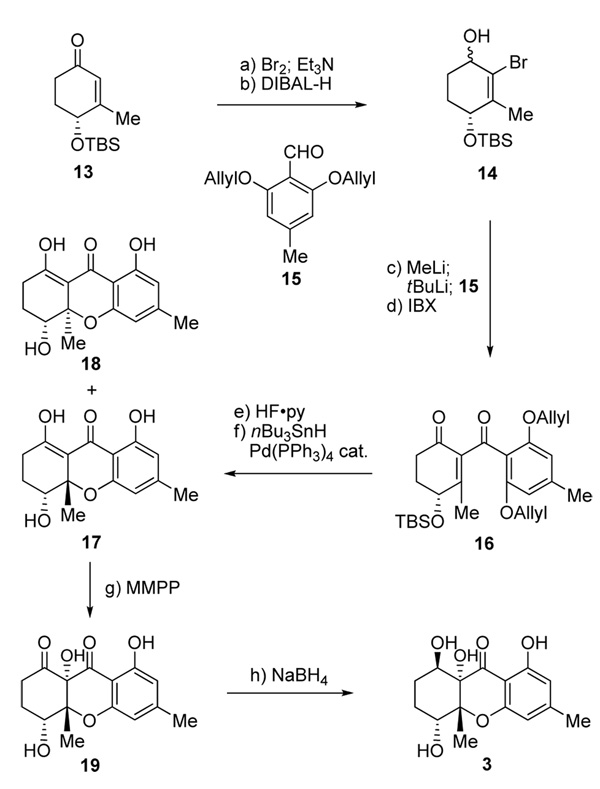
Scheme 2.
Total synthesis of diversonol (3). Reagents and conditions: a) Br2 (1.05 equiv), CH2Cl2, 0 °C, 5 min; then Et3N (1.5 equiv), 0 °C, 5 min, 90 %; b) DIBAL-H (1.0 M in hexanes, 1.5 equiv), THF, −78→ −40 °C, 30 min, 95 %, ca. 1:1 dr; c) MeLi (1.6 M in ether, 1.1 equiv), ether, −78 °C, 15 min; then tBuLi (1.7 M in pentane, 2.2 equiv), −78 °C, 15 min; then 15 (1.5 equiv), −78→ −40 °C, 40 min; d) IBX (3.0 equiv), DMSO, 23 °C, 1 h, 72 % over two steps; e) HF•py/THF (1:5), 23 °C, 12 h, 96 %; f) nBu3SnH (2.2 equiv), AcOH (2.2 equiv), Pd(PPh3)4 (0.05 equiv), benzene, 23 °C, 1 h, 90 %, 17:18 ca. 2:1 dr; g) MMPP (0.75 equiv), EtOH, 23 °C, 30 min; h) NaBH4 (1.0 equiv), MeOH/CH2Cl2 (1:1), −78 °C, 15 min, 73 % over two steps. MMPP=magnesium monoperoxophthalate.