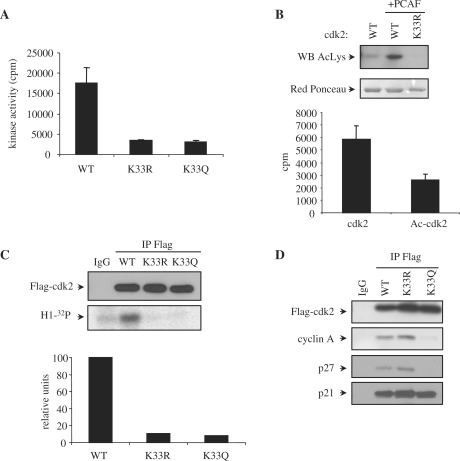
Figure 8.
Acetylation of cdk2 impairs its kinase activity both in vivo and in vitro. (A) In vitro kinase assays were performed using 400 nM of purified recombinant GST-cdk2WT, K33R or K33Q together with 400 nM of cyclin A. Kinase activity ± SE was represented in the graph. (B) GST-cdk2 WT and K33R were expressed in bacteria co-expressing 6His-PCAF. After purification of GST-cdk2 WT and K33R, they were analysed by WB with anti-Acetyl-K (top panel). Red Ponceau staining of the proteins is shown in the bottom panel as a loading control. GST-cdk2 WT purified in the absence or presence (Ac-cdk2) of PCAF were tested for in vitro kinase activity. 400 nM of the proteins were incubated with 400 nM of purified GST-cyclin A in the presence of histone H1 as a substrate and [32P]ATP as a cofactor. Kinase activity ± SE was represented in the graph. (C) HeLa cells were transfected with Flag-cdk2 WT, K33R or K33Q. Cell extracts were subjected to IP with anti-Flag or IgG as a control. A WB performed with anti-Flag is shown in the top panel. Kinase assays were also performed with the immunoprecipitates and their kinase activity was quantitated with a PhosphorImager (bottom panel). Normalization of cdk activity with respect to the amount of immunoprecipitated cdk2 is shown in the graph. (D) 293-T cells were transfected with Flag-cdk2WT, K33R or K33Q. Cell extracts were subjected to IP with anti-Flag or IgG as a control. A WB performed with anti-Flag is shown in the upper panel. Interaction of the different cdk2 forms with cyclin A, p21 and p27 was analyzed by WB.