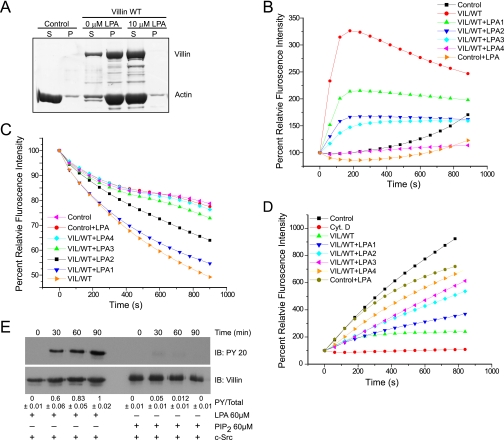
FIGURE 2.
LPA inhibits the actin regulatory functions of villin. A, full-length villin (1.0 μm) was incubated with F-actin in the absence or presence of LPA (10 μm). The samples were centrifuged at 10,000 × g for 15 min, and actin distribution in the supernatant (S) and pellet (P) fractions was analyzed by 10% SDS-PAGE and GelCode Blue staining. Control refers to F-actin filaments in the absence of villin. This experiment is representative of four with similar results. B, LPA inhibits actin nucleation by villin. Pyrene-labeled G-actin (6 μm) was incubated with full-length villin protein (VIL/WT; 60 nm) in the absence or presence of different concentrations of LPA (LPA1, 60 μm; LPA2, 120 μm; LPA3, 200 μm; LPA4, 240 μm) in polymerization-inducing buffer. Fluorescence intensity was measured over time. Control and Control+LPA (240 μm) represent the polymerization of actin in the absence of villin. Values represent the mean of three independent experiments. C, LPA inhibits the actin-depolymerizing function of villin. The effect of LPA on actin depolymerization was recorded using recombinant full-length villin protein (VIL/WT) and different concentrations of LPA as described above. Values represent the mean of three independent experiments. D, LPA inhibited actin-capping activity of villin. To measure the actin-capping activity of villin in the absence or presence of LPA, 1.4 μm G-actin was nucleated by F-actin (290 nm) seeds in the absence (Control) or presence of VIL/WT. Different concentrations of LPA were used as described above. Values represent the mean of three independent experiments. Cyt D., cytochalasin D. E, recombinant villin was phosphorylated in vitro by c-Src in the absence or presence of LPA (60 μm) or PIP2 (60 μm) over a period of 90 min. This is a representative of three experiments with similar results. Densitometric analysis gave a phosphovillin:villin (PY/Total) ratio of 0.6 ± 0.06; 0.83 ± 0.05; and 1.0 ± 0.01, which corresponds to 60, 83, and 100% (n = 3; p < 0.01) increase in villin phosphorylation at 30, 60, and 90 min, respectively. IB, immunoblot. Tyrosine phosphorylation of villin was identified by Western analysis using a phosphotyrosine antibody (PY 20). Blots were stripped and reprobed for villin.