FIGURE 9.
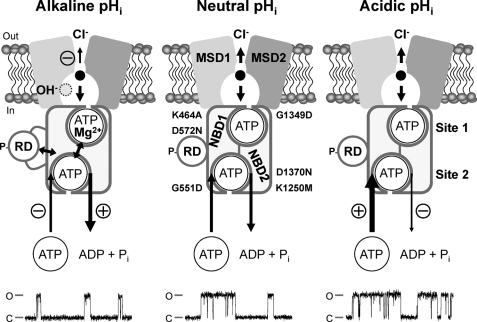
Multiple mechanisms of CFTR regulation by pHi. The simplified models show an open CFTR Cl− channel with a phosphorylated RD and NBDs assembled to form a head-to-tail dimer with ATP molecules bound at ATP binding sites 1 and 2. Each ATP binding site is formed by the Walker A and B motifs of one NBD and the LSGGQ motif of the other NBD. The locations of the site-directed mutations examined in this study are shown. The models show the direct effects of H+ and OH− ions on the function of the individual domains of CFTR at alkaline, neutral, and acidic pHi. Single-channel recordings are shown beneath each model. For the purpose of illustration, the recordings have been inverted, and upward deflections correspond to channel openings. MSD, membrane-spanning domain; P, phosphorylation of the RD; Pi, inorganic phosphate. In and Out denote the intra- and extracellular sides of the membrane, respectively, whereas C and O indicate the closed and open-channel states, respectively. The long arrows represent the regulation of CFTR channel gating by ATP-driven NBD dimerization; arrow thickness and symbols indicate reaction speed (thick (+), fast (potentiation); thin (−), slow (inhibition)). Double-headed arrows denote interactions between the NBDs and RD and cross-talk between ATP-binding sites 1 and 2. Short arrows within the CFTR pore denote Cl− ion flow; arrow thickness and symbols indicate ease of flow (thick, unobstructed; thin (−), obstructed). See “Discussion” and Refs. 2, 3, and 25 for further information.