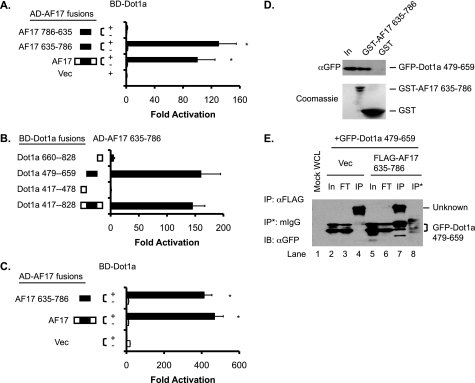
FIGURE 2.
The AF9-interacting domain in Dot1a is capable of and sufficient for interaction with AF17 in vitro and in vivo. A, shown is a yeast two-hybrid analysis revealing that Dot1a interacts with full-length human AF17 and mouse AF17 635–786 but not with the latter when it is placed in a reverse orientation (AF17 786–635). The bars represent the average -fold activation of the LacZ reporter from three independent experiments (n = 3). *, p < 0.05 versus Vec (pGADT7). B, as in A, mapping by yeast two-hybrid analysis showing that the AF9-interacting domain in Dot1a (aa 479–659) is capable of and sufficient for interacting with AF17; n = 3. C, shown is a mammalian two-hybrid assay confirming that Dot1a interacts with AF17 or AF17 635–786 in 293T cells. The bars represent the average -fold activation of a GAL4-dependent luciferase reporter from three independent experiments (n = 3). *, p < 0.05 versus BD-Dot1a plus Vec (pCMV-AD). D, shown is a GST pulldown assay showing that specific domains in Dot1a and AF17 are responsible for the interaction. GST and GST-AF17 635–786 were purified from E. coli and incubated with whole-cell lysates of 293T cells expressing GFP-Dot1a 479–659. Input (In) of the lysates (5%) and proteins bound to Glutathione-Sepharose 4B beads were examined by immunoblotting with a mouse anti-GFP antibody. The inputs of GST and GST-AF17 were analyzed by Coomassie staining (lower panel). E, co-immunoprecipitation assay demonstrating the interaction between Dot1a and AF17. Whole-cell lysates (WCL) of 293T cells transiently expressing GFP-Dot1a 479–659 with or without FLAG-AF17 635–786 were immunoprecipitated (IP) with a mouse anti-FLAG antibody or equal amount of normal mouse IgG (mIgG). Immunoprecipitated proteins were eluted from the Protein A/G-agarose beads and subjected to IB analysis with the mouse anti-GFP antibody. In, input (2%); FT, flow-through (2%); Vec, vector.