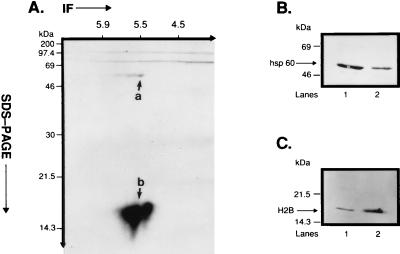
Figure 2.
Immunoprecipitation of plasma membrane-associated phosphoproteins by mAb II-13 and immunoblotting of p60 and p18. (A) Isolated CEM-SS T cell plasma membrane fragments (60 μg protein) were phosphorylated in vitro by 50 nM PKA C-subunit in the presence of γ-[32P]ATP. In vitro phosphorylated fragments were immunoprecipitated with mAb II-13, and the immunoprecipitate was resolved on a 15% 2-D SDS/PAGE. Coimmunoprecipitation of phsp60 and pp18 was observed on the autoradiogram. Also notable was that both phsp60 and pp18 were comprised of isoforms whose pI values varied between ≈5.5 and 5.8. Differences in the relative intensity of phosphorylation of phsp60 and pp18 are addressed in Discussion. (B) Isolated CEM-SS T cell plasma membrane fragments (lane 1, 60 μg protein) and 1 μg of rhsp60 (lane 2) were run on a 15% 1-D SDS/PAGE and were immunoblotted with mAb II-13. (C) Isolated CEM-SS T cell plasma membrane fragments (lane 1, 60 μg protein) and 2 μg of purified human H2B (lane 2) were run on a 15% 1-D SDS/PAGE and were immunoblotted with affinity-purified polyclonal anti-H2B Ab.