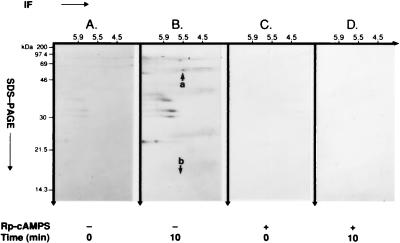
Figure 4.
cAMP-dependent, PKA-catalyzed phosphorylation of hsp60 in intact CEM-SS T cell isolated plasma membrane fragments. After loading with [32Pi]orthophosphate for 3 hr, cells were exposed to 2.5 mM bt2-cAMP in the presence of 200 μM isobutylmethylxanthine and 10 μM okadaic acid for 0 min and 10 min. Plasma membrane fragments then were isolated, and phsp60 was immunoprecipitated with mAb II-13. Immunoprecipitated pp60 was analyzed by 15% 2-D SDS/PAGE. (A) At 0 min, shows the absence of basal phosphorylation of hsp60. Note that other, unidentified phosphoproteins are coimmunoprecipitated with hsp60, but pH2B is not observed. (B) At 10 min, shows immunoprecipitated phsp60 (arrow a). Although other, unidentified phosphoproteins are coimmunoprecipitated with phsp60, pH2B is not observed (arrow b). (C) At 0 min with 500 μM Rp-cAMPS. (D) At 10 min with 500 μM Rp-cAMPS blocks cAMP-dependent, PKA-catalyzed phosphorylation of hsp60.