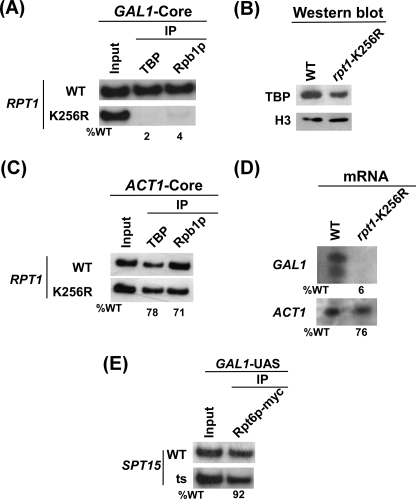
FIGURE 4.
19 S ATPase activity is essential for formation of the PIC assembly at the GAL1 core promoter. A, shown is analysis of TBP and RNA polymerase II recruitment to the GAL1 core promoter in the wild type and rpt1-K256R point mutant strains. Both the wild type (WT) and point mutant strains were grown and cross-linked as in Fig. 2A. Immunoprecipitations were performed using polyclonal antibodies against TBP and the mouse monoclonal antibody 8WG16 (Covance) against the C-terminal domain of the RNA polymerase II large subunit (Rpb1p). B, shown is Western blot analysis. The wild type and rpt1-K256R mutant strains were grown as in panel A. The whole cell extracts from both the wild type and mutant strains were prepared as in the ChIP assay. The whole cell extracts were analyzed by Western blot assay using the anti-TBP and anti-histone H3 antibodies against TBP and histone H3, respectively. C, analysis is shown of TBP and RNA polymerase II recruitment to the ACT1 core promoter in the wild type and rpt1-K256R point mutant strains. The wild type and mutant strains were grown and cross-linked as in panel A. Immunoprecipitation was performed as in panel A. The primer pair located at the ACT1 core (“Experimental Procedures”) was used for PCR analysis of the immunoprecipitated DNA samples. D, transcription is shown. Total cellular RNA was prepared from the wild type or the mutant strain, and the mRNA levels from the GAL1 and ACT1 genes were quantitated as described previously (64). E, TBP is not required for recruitment of the 19 S base to the GAL1 UAS. Both the wild type and TBP (SPT15) ts mutant strains expressing Myc-tagged Rpt6p were grown, cross-linked as in Fig. 3A. Immunoprecipitation was carried out as in Fig. 3A.