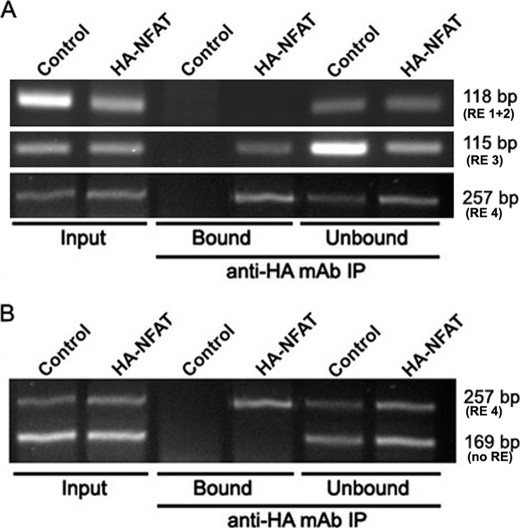
FIGURE 6.
ChIP of endogenous hTERT promoter. MCF7 cells were cotransfected with expression vectors encoding HA-NFAT1 and a constitutively active form of calcineurin. A ChIP assay was performed with 5 μg of anti-HA monoclonal antibody (12CA5). As a negative control, the same experiment was performed with MCF7 cells cotransfected with the empty version of the HA-NFAT1 expression vector. Immunoprecipitated DNA was analyzed by PCR using hTERT promoter-specific primers that amplify three different sequences containing putative NFAT-binding sites. PCR products were visualized on a 3% agarose gel. A, illustration of DNA PCR amplification sequences obtained in the different ChIP fractions and corresponding to the 118-, 115-, and 257-bp bands, which contain the RE 1 + 2 (−1225 and −1220), RE 3 (−775), or RE 4 (−40) responsive elements, respectively. B, illustration of the duplex PCR amplification in the different ChIP fractions. Immunoprecipitated DNA was analyzed by PCR using hTERT promoter-specific primers that amplify a 257-bp region containing the putative −40 (RE 4) and no others. To verify the specificity of the results, a control was performed using hTERT promoter-specific primers that amplify a 169-bp region lacking any putative NFAT-binding site (no RE). “Input” bands were obtained from DNA purified from chromatin not yet immunoprecipitated, “Bound” corresponds to DNA co-immunoprecipitated with HA-NFAT1 proteins, and “Unbound” to DNA in the supernatant prior to elution.