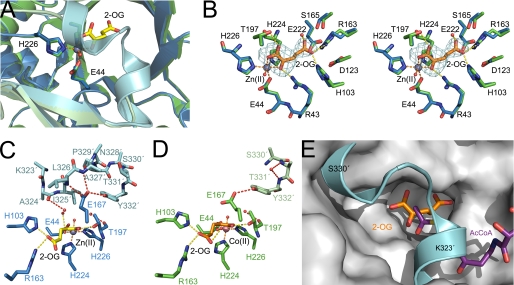
FIGURE 5.
The lid motif of SpHCS. A, structural superimposition of the SpHCS·2-OG complexes with the open lid motif (monomer A is green, and monomer B is pale green) and the closed lid motif (monomer A is blue, and monomer B is cyan). The 2-OG and Zn(II) ion of the closed lid structure are depicted in yellow carbons and gray, respectively. B, shown is a stereoview of the active site of SpHCS·2-OG open lid complex (green carbon atoms with 2-OG rendered with orange carbons) overlaid with the SpHCS·2-OG closed lid complex (blue carbons). Electron density for the Fo − Fc-simulated annealing omit map (contoured at 2.0 σ) corresponding to the 2-OG in the open lid complex is shown in cyan. The orange and yellow dashes represent coordination of the Zn(II) ion and hydrogen bonding to 2-OG, respectively. C and D, shown are comparisons of the active site and lid motif of the SpHCS·2-OG open and closed lid complexes. Monomer A (blue carbons) and B (cyan carbons) of the closed lid complex are depicted with the Zn(II) ion (gray) and 2-OG (yellow carbons) (C), whereas monomer A (green carbons) and B (light green carbons) of the open lid complex are illustrated with the Co(II) ion (pink) and 2-OG (orange carbons) (D). Dashed lines denote the coordination of the metal ion (orange), hydrogen bonding to 2-OG (yellow), and hydrogen bonding within the lid motif (red). Residues in the lid motif that lack interpretable side-chain electron density are modeled as alanines, whereas the side chain of Tyr-332 in the open lid complex (D) is modeled with an occupancy of 0.5. E, shown is a model of a ternary complex of SpHCS bound to 2-OG (orange carbons) and AcCoA (violet carbons). The lid motif of the SpHCS·2-OG closed lid complex (cyan) is superimposed on the gray surface of the SpHCS·2-OG open lid complex.