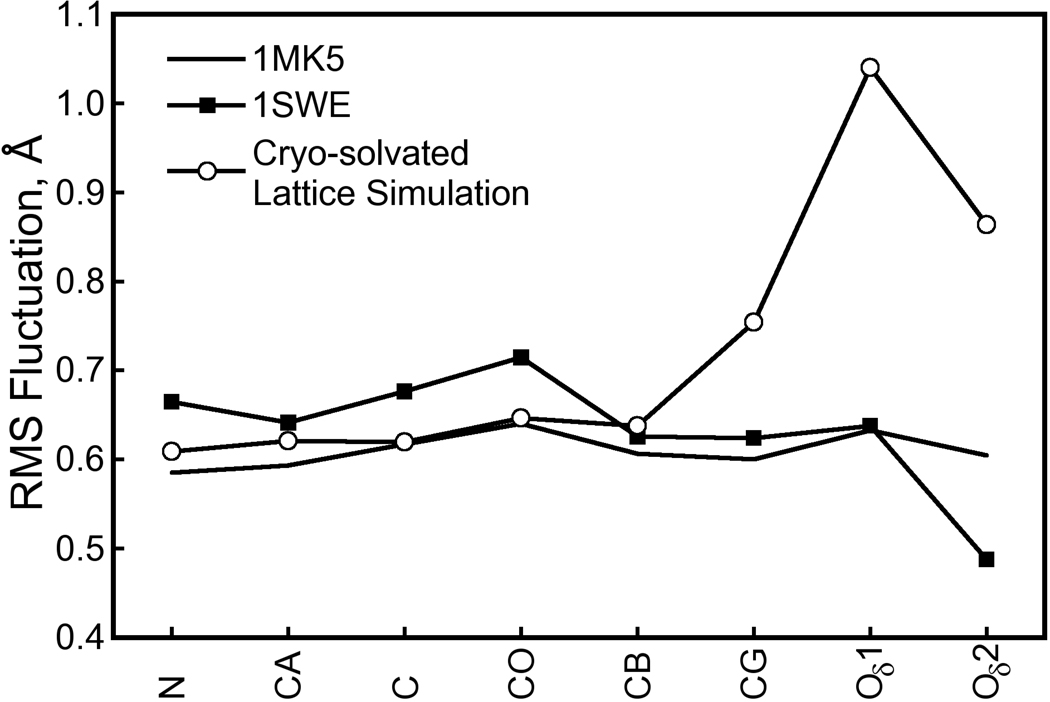
Figure 16. Atomic RMS fluctuations for Asp128 heavy atoms in crystal structures and the cryo-solvated lattice simulation.
Fluctuations were computed for heavy atoms of the streptavidin Asp128 residue as described in Figure 11. The Asp128 residue is not in any of the streptavidin flexible loop regions and has low mobility at both cryogenic and room temperatures. However, in our cryo-solvated lattice simulation, we observe the Asp128 carboxylate group to move away from the biotin N1 atom, breaking the hydrogen bond between these groups and possibly allowing the intercalation of a water molecule (see Figure 14). Whereas our (room temperature) simulation is in good agreement with X-ray data collected at either temperature in terms of the Asp128 backbone fluctuations, the motion of the Asp128 side chain raises the observed atomic fluctuations well above those in either X-ray structure. This result, and others noted in the text, may indicate the need for a more sophisticated hydrogen bonding potential in molecular simulations.