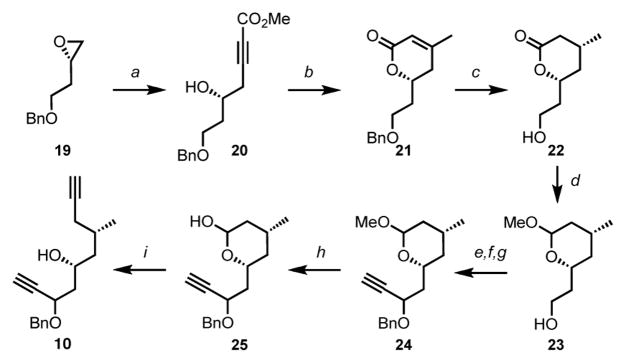
Scheme 4. Synthesis of the diyne precursor 10a.
aConditions: (a) Methyl propiolate, n-BuLi, BF3·Et2O, THF, 93%; (b) CuI, MeLi, THF then AcOH, PhH, 94%; (c) Pd(OH)2, H2, EtOAc, 97%; (d) DIBAL-H, CH2Cl2 then Dowex 50W × 8, MeOH, 99%; (e) TEMPO, NaOCl, KBr, NaHCO3, CH2Cl2/H2O, 97%; (f) Ethynylmagnesium bromide, THF, 77%; (g) NaH, BnBr, DMF, 96%; (h) AcOH, H2SO4, H2O, 82%; (i) Dimethyl-1-diazo-2-oxopropylphosphonate, K2CO3, MeOH, 57% (69% brsm).