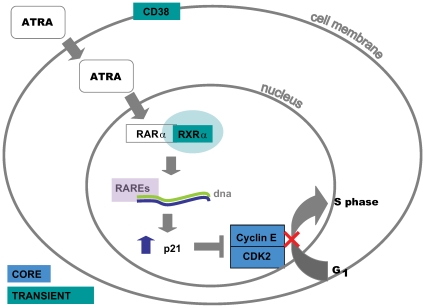
Figure 3. ATRA signaling pathway.
ATRA is able to diffuse freely across the cell membrane. A pair of cellular retinoic acid binding proteins act as cell surface receptors for retinoids however these have been shown to be dispensable in retinoic-acid signaling [21]. ATRA binds to a family of nuclear hormone receptors called retinoic-acid receptors (RARs). There are three subtypes of the RAR family, these are encoded by different genes and denoted RAR-α, RAR-β, RAR-γ. Collins et al [22] demonstrated that in HL-60 cells, ATRA induced granulocytic differentiation by binding RAR-α directly. RAR-α binds to specific cis-acting DNA sites, known as retinoic-acid response elements (RAREs). These RAREs are located in the promoter sequences of specific genes that are targets of RAR-α. In order to bind DNA efficiently, RARs must however form heterodimers with a second family of nuclear hormone receptors, the retinoid X receptors (RXRs), of which there are three subtypes: RXR-α, RXR-β, RXR-γ. Both RXRs and RARs function as ligand-dependent transcription factors. One of the RAR-target genes whose expression is upregulated is the cell cycle protein p21 [23]. p21 inhibits the cyclin dependent kinase complex Cyclin E and CDK2. In this way, ATRA induces cell cycle arrest at the G1 to S phase transition checkpoint.