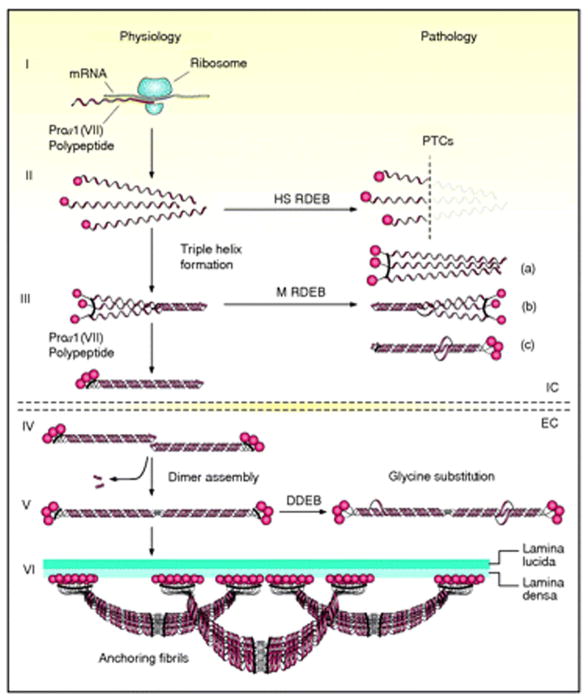
Figure 3.
Schematic presentation of the synthesis of pro-α1(VII) collagen polypeptides and their assembly into anchoring fibrils under physiological conditions (left side of the figure) and perturbations in these processes leading to dystrophic epidermolysis bullosa (right side). Within the intracellular (IC) space of cells, such as keratinocytes and fibroblasts, pro-α1(VII) polypeptides are synthesized on ribosomes (I). Three polypeptides associate through their carboxy-terminal ends, and their collagenous domains fold into a characteristic triple-helical conformation (II and III). After secretion into the extracellular (EC) space, triple-helical typeVII collagen molecules form anti-parallel dimers (IV), and after proteolytic removal of a part of the carboxy-terminal end, the dimer assembly is stabilized by intermolecular disulfide bonds (V). Subsequently, a large number of dimer molecules laterally assemble in register to form cross- striated, centro-symmetric anchoring fibrils (VI). The amino-terminal non-collagenous globular domains (NC-1) attach to the extracellular macromolecules of the lamina densa, stabilizing the association of the lower part of the dermo-epidermal basement membrane to the underlying dermis. Mutations in the COL7A1 gene can result in premature termination codons (PTCs), manifesting with the severe Hallopeau-Siemens (HS)-type recessive dystrophic epidermolysis bullosa (RDEB) when present in both alleles. Recessive missense mutations can interfere with chain assembly (a), triple helix formation (b), or stability of the triple-helix (c), resulting in mild (mitis, M) non-HS-RDEB. Glycine substitutions in the collagenous domain destabilize the triple helix and can result through dominant-negative interference in dominantly inherited DEB (DDEB). (Adapted from Varki R, Sadowski S, Uitto J, et al. Epidermolysis bullosa. II. Type VII collagen mutations and phenotype/genotype correlations in the dystrophic subtypes. J Med Genet 2007; 44: 181–92., with permission).