Figure 1.
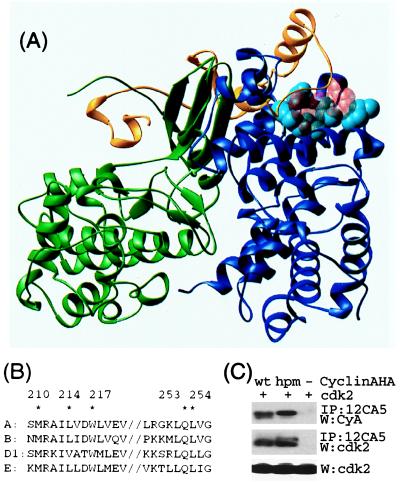
Structural basis for interactions with cyclin A. (A) Crystal structure of cyclin A (blue) -cdk2 (green) -p27 (yellow) (35). The residues mutated in this report are in light-blue space-filling representation. The RXL motif (R30, L32, and F33) involved important in p27–cyclin interactions is shown in transparent red space-filling representation. (B) The sequence in cyclin A that contacts p27 is conserved among a number of mammalian cyclins. Residues mutated in this report are marked with ∗. (C) The hydrophobic patch mutant binds cdk2 when immunoprecipitated from lysates of U2OS cells cotransfected with plasmids expressing HA-tagged cyclin A and cdk2. A representative experiment is shown. In a series of similar experiments, the amount of cdk2 present in 12CA5 immunoprecipitates is proportional to the amount of either HA-tagged cyclin A or cyAhpm.