Abstract
A variety of neuroimaging tools are now available for use in studying neurodevelopment. In this paper we focus our attention on one such tool – the event-related potential (ERP). We begin by providing an overview of what ERPs are, their physiological basis, how they are recorded, and some constraints on their use. We then provide an abbreviated glossary of ERP components; that is, what processes are reflected in ERPs. We conclude by summarizing two areas of atypical development that have benefited from this method: children experiencing early psychosocial neglect, and children diagnosed with autism. We conclude by offering recommendations for future research.
In 1997, Bloom and Nelson lamented the paucity of research elucidating the neural bases of behavioral development. Over the ensuing 10+ years, the field of developmental cognitive neuroscience has gained considerable traction, with entire single-authored (1, 2) and edited volumes (3, 4, 5) dedicated to this field, along with special issues of journals (e.g., Journal of Cognitive Neuroscience; Neuropsychologia: Developmental Review). Among the many reasons for the progression of this field has been the advances made in neuroimaging. Specifically, the refinement of existing tools (e.g., event-related potentials; functional Magnetic Resonance Imaging) and the development of new tools (e.g., functional Near Infrared Spectroscopy) has now made possible the ability to examine the neural correlates of cognitive and emotional development.
In this paper we focus our attention on one particular tool – the recording of the event-related potential (ERP). The ERP reflects the brain's electrical activity recorded from electrodes placed on the scalp surface and can be utilized across the entire lifespan, thereby permitting one to use the same methodological tool and dependent measure across a broad range of ages.
What is the Event-Related Potential?
ERPs represent the synchronous activation of electrical fields associated with the activity of large populations of neurons. This activity volume conducts to the scalp surface, and is configured in such a way that their individual electrical fields summate to yield a dipolar field (a field with positive and negative charges between which current flows).
ERPs reflect changes in the brain's electrical activity in response to a discrete stimulus or event. They are typically collected during the presentation of stimuli that repeat many times. Recording generally begins 100 or more milliseconds before a stimulus is presented and then continues for 500 to 2000 msec after the stimulus has terminated. Each “trial” or epoch is generally averaged in order to eliminate background noise that is not related to the stimulus of interest. As a result of averaging, the noise theoretically goes to 0 and the signal emerges from the background, yielding a series of positive and negative deflections – so-called “components” - in the ongoing EEG, each of which is presumed to reflect a different neural and perceptual/cognitive operation. Due to the high temporal resolution (on the order of milliseconds), ERPs are well suited to index changes in the mental chronometry of a given neural response.
How does one collect ERPs?
Until recently, one typically recorded from 32 or fewer electrodes, mostly due to limitations in how electrodes were fixed to the scalp and in the size of the amplifiers. However, the field has increasingly moved to higher-density arrays of electrodes, made possible by new means of applying electrodes and the miniaturization of the hardware. As a result, it is not uncommon for many investigators to record from 64, 128, or even 256 electrodes. The advantages to these larger arrays are multiple. First, the greater spatial sampling permits one to identify components that might have eluded capture with smaller arrays, where the inter-electrode distances were greater. Similarly, greater spatial sampling permits one to distinguish one component from another based in part on scalp topography. Third, great advances have been made in source modeling/localization, which depend critically on the use of many electrodes. A final benefit, that has little to do with science per se, is that some dense array systems are quick and easy to put on, such as the EGI electrode net. As a result, this makes possible the ability to test, using many electrodes, infants or other difficult-to-test children.
What processes have been investigated using ERPs?
Topics that have received the most attention in ERP research include recognition memory, attention, working memory, executive functions, auditory and visual sensory processing, face processing, and language processing. In this section, we review a selection of the processes that have been examined in developmental populations using ERPs, and some of the associated ERP components, with an emphasis on those that are most relevant for a developmental approach to studying psychiatric disorders. We also include a glossary of these and other ERP components in this section (see Table 1).
Table 1.
Glossary of some ERP components commonly examined in studies of typical and/or atypical development, as well as some relevant adult components. Components are typically labeled according to the polarity of the deflection (i.e., P = positive; N = negative), and the peak latency of the component in milliseconds (e.g., N170 peaks approximately 170 ms following the presentation of a visual stimulus). Alternatively, components are sometimes labeled based on the topography of the ERP waveform (e.g., the auditory N2 is the second negative deflection observed in response to an auditory stimulus). Finally, the names of other components are derived from their apparent functional significance (e.g., Error-Related Negativity is observed following an errant motor response) or their scalp topography (e.g., Left Anterior Negativity).
| Process | Component | Ages Observed | Function | Scalp distribution, Timing | Notes |
|---|---|---|---|---|---|
| Auditory Sensory | Mismatch Negativity (MMN) | Birth onward | Obligatory auditory discrimination and/or sensory memory. | Fronto-central, 100 – 250 ms | Plotted as a difference wave (deviant stimulus response minus standard stimulus response) |
| P50 / P1 Repetition Suppression | Early adolescence onward | Reduction of amplitude following repetition, indexes sensory inhibition | Vertex, ∼50 ms in adults | This function may be observable in neonates. May also be observable in the visual domain. | |
| N2 | Infancy onward | Stimulus complexity, tone processing, speech vs non-speech in children and adults | Fronto-Central, 150 - 250 ms in adults; ∼250 - 350 ms in children | ||
| --------------- | ---------------- | ------------ | ----------------- | --------------- | ----------------- |
| Visual Sensory | P100 | Birth / infancy | Visual pattern reversal, visual thresholds, visual discrimination thresholds | Occipital, ∼100 ms in adults; 250 – 300 ms in neonates | |
| C1 | Adulthood | Initial cortical processing in V1 (∼ 56 ms) | Occipital, ∼60 ms | ||
| P1 | Infancy, adulthood | Under unique circumstances, may index magnocellular visual pathway functioning | Occipital, ∼100 ms | ||
| N1 | Birth / infancy, adulthood | Under unique circumstances, may index parvocellular visual pathway functioning | Occipital, 65 – 85 ms | ||
| --------------- | ---------------- | ------------ | ----------------- | --------------- | ----------------- |
| Face Processing | N290 | Infancy and early childhood | Sensory-perceptual processing of faces | Occipital-Temporal, ∼290 ms | See also N170, P400 |
| P400 | Infancy and early childhood | Sensory-perceptual and early cognitive processing of faces | Occipital-Temporal, ∼400 ms | See also N290, N170 | |
| P1 / P100 | Birth onward | Face processing during childhood, face inversion during childhood | Occipital-Temporal, ∼100 ms in adults | Significant developmental changes, sometimes indexing face processing | |
| N170 / N1 | Middle childhood onward | Sensory-perceptual processing of faces, perceptual experience | Occipital-Temporal, ∼170 ms | See also VPP, P100, N290, P400 | |
| Vertex Positive Potential (VPP) | Adulthood | Sensory-perceptual processing of faces | Centro-Frontal, ∼170 ms | Highly similar functionally to the N170 | |
| --------------- | ---------------- | ------------ | ----------------- | --------------- | ----------------- |
| Memory / Attention | Negative Central (Nc) | Infancy and childhood | Attention, recognition memory | Fronto-Central, ∼550 ms | Auditory, visual, and cross-modal. Also relevant for face processing and cognition |
| Negative Slow Wave (NSW) | Recognition memory | Fronto-Central, ∼1000 – 1500 ms | Auditory, visual, and cross-modal | ||
| Positive Slow Wave (PSW) | Updating of memory representations | Fronto-Central, ∼650 – 1250 ms | Auditory, visual, and cross-modal | ||
| P3a | Middle childhood onward | Attentional engagement, sensory working memory | Midline with frontal maximum, 250 – 500 ms | Time-locked to a target stimulus in oddball paradigms. Part of the P300 / P3 / LPC. See also P3b. | |
| P3b | Middle to late childhood onward | Context updating relevant to memory storage | Midline with parietal maximum, 350 – 550 ms | Time-locked to a target stimulus in oddball paradigms. Part of the P300 / P3 / LPC. See also P3a. | |
| --------------- | ---------------- | ------------ | ----------------- | --------------- | ----------------- |
| Language | N200-400 | Toddlerhood | Word familiarity, hemispheric specialization for words | Temporal-Parietal, 200 – 400 ms | Analyzed as mean amplitude differences |
| N400 | Early childhood onward | Semantic context match / mismatch, semantic integration | Centro-Parietal, ∼400 ms | An N400-like response has been observed in toddlers | |
| P600 / Syntactic Positivity Shift (SPS) | Early childhood, Adulthood | Syntactic violations, rule-based violations | Centro-Parietal, ∼600 ms | See also LAN / ELAN | |
| Left Anterior Negativity (LAN) / Early Left Anterior Negativity (ELAN) | Middle childhood onward | Syntactic structure violations, rule-based sequence violations | Left Frontal, ELAN ∼200 ms, LAN 300 – 700 ms | See also P600 / SPS | |
| --------------- | ---------------- | ------------ | ----------------- | --------------- | ----------------- |
| Executive Functioning | Error-Related Negativity (ERN) / Error Negativity (Ne) | Late childhood onward | Error monitoring, response evaluation | Centro-Medial, 50 – 150 ms | Time-locked to subjects' motor / button box response. See also N2 |
| N2 | Late adolescence onward | Response inhibition | Fronto-Central, 200 – 300 ms | Time-locked to correct responses on incongruent trials, or in go / no-go tasks | |
| Error Positivity (Pe) | Adolescence onward | Cognitive / emotional evaluation or response errors | Centro-Parietal, 200 – 400 ms | Time-locked to subjects' motor response errors. See also ERN and N2 | |
| Contingent Negative Variation (CNV) | Late childhood onward | Stimulus evaluation, motor and cognitive preparation | Fronto-Central, 400 – 800 ms | Occurs between a warning stimulus and a stimulus requiring a response | |
| --------------- | ---------------- | ------------ | ----------------- | --------------- | ----------------- |
Sensory Processing
An auditory sensory component relevant to developmental psychopathology research is the Mismatch Negativity (MMN). The MMN is a negative-going component recorded from centro-frontal electrodes approximately 175 ms following a rarely-presented auditory stimulus, reflecting an automatic change-detection response (6). This early obligatory component is present from birth through adulthood (7), is typically presented and analyzed as a difference wave (i.e., standard stimulus waveform minus oddball stimulus waveform), and is robust to cognitive state to such an extent that it not only occurs when participants are ignoring the stimuli (8) but also when they are engaged in a cognitively demanding task in another modality (e.g., 9). The MMN reflects the earliest stage of obligatory auditory attention, and is generally believed to be the outcome of a mechanism that compares current auditory input to memory traces from previous auditory inputs and signals (e.g., 10; but see also 11). The MMN has already been utilized to examine low-level auditory sensory abilities in infants, children, and adults with a variety of medical and psychiatric disorders (e.g., 12, 13, 14, 15, 16, 17).
Face Processing
Human faces provide critical signals for normal social and communicative interaction, and face processing and the neural and perceptual mechanisms that underlie it are directly or indirectly relevant for a wide variety of psychiatric disorders. The face-sensitive adult N170 component is a negative deflection recorded from electrodes over occipital-temporal cortex that peaks at approximately 170 ms following the presentation of a picture of a face or object. Decades of research indicate that the N170 exhibits larger amplitude and shorter latency responses to faces than to a variety of other stimuli (18). The N170 also exhibits face inversion effects, characterized by larger amplitude and/or longer latency responses to inverted relative to upright faces but not for inverted relative to upright objects (19). In terms of its neural sources, converging evidence suggests that the N170 reflects specialized activity in several regions of the occipital and temporal lobes that are involved in face processing (18, 20, 21, 22).
The N170 has been studied extensively as a marker for the specialized neural and perceptual mechanisms associated with the early stages of face processing, and recent evidence-suggests that the face-sensitive responding of the N170 may index a collection of specialized early-stage neural and perceptual mechanisms that are unique to the recognition and identification of faces as a salient and important visual stimulus (23, 24, 25, 26). Most notably, the results of several studies suggest that the large amplitude of the N170 component in response to faces compared with objects may reflect the extensive long-term experience we have with identifying and discriminating faces from one another (26, 27).
There are considerable functional differences between the face-sensitivities of N170 responses in children and adults (see 28 for review). However, the N170 is observable in children as young as 4 years of age, and the electrophysiological processing of faces becomes adult-like during adolescence. Additionally, researchers have identified two infant ERP components, the N290 and the P400, which may represent developmental precursors to the adult N170 (29). Like the N170, these components are recorded from electrodes over occipital-temporal cortex, and are sensitive to faces on a number of dimensions. Specifically, the N290 has been observed to elicit larger amplitude responses to human faces relative to monkey faces or matched visual noise stimuli (30, 31, 32), P400 latency is shorter for faces than for other objects (33), and the N290 and P400 both exhibit face inversion effects with larger amplitude responding for upright compared with inverted faces (30, 31).
We also recently found that the occipital-temporal P400 (in infants) and N170 (in adults) were similar in that they both exhibited larger amplitude responses to fear faces versus happy or neutral faces (34). These findings complement previous research in which we showed that a later ERP component recorded from electrodes over frontal cortex (Nc) also exhibited increased amplitude responses to fearful versus happy faces (35, 36, 37). We have also shown that the Nc component indexes familiarity in face processing, in that it is larger for familiar as compared to unfamiliar faces in the first year of life (38). Taken together, these data suggest that increased neural resources are allocated to the processing of a variety of face-related processes at the perceptual (N290/P400) and early cognitive stages (Nc), and that several important aspects of face processing at these two stages are already indexed differentially in ERP components in infancy. Furthermore, developmental changes observed in face-sensitive ERP components are believed to reflect the effects of experience in shaping the neural systems that underlie face processing. Therefore, studying the development of face-related ERP components in psychiatric populations is an especially promising area of current and future research.
Memory and Attention
Mnemonic and attentional ERPs are relevant for the study of psychiatric disorders in at least two respects. First, abnormalities of memory and/or attentional processes are part and parcel of many psychiatric disorders. Therefore, the use of ERPs can shed light on the nature and timing of the neural abnormalities that underlie them, as well as their developmental course. Second, ERPs that reflect memory or attention have proven to be invaluable tools for exploring new ground in a variety of cognitive domains in both typical and atypical development (e.g., language, face processing).
The Nc component observed in infants and children is one of the most studied developmental ERP components. This negative deflection is recorded from electrodes over frontal cortex, exclusively to visual stimuli, and is involved in both memory and attention. The Nc is present at birth, and initial studies showed that it is consistently larger in amplitude and/or longer in latency in response to infrequently presented (e.g., 25%) as compared to frequently presented (e.g., 75%) stimuli from three months of age onward (39, 40, 41). As noted earlier, the Nc has also been observed to be of larger amplitude in response to fearful versus happy facial expressions (35). Based on these and other data, it has been suggested that the Nc may specifically index the allocation of attentional resources to interesting or salient stimuli (39, 42, 43).
In addition to the role it may play in indexing attentional mechanisms, there is evidence that the Nc component also indexes mnemonic mechanisms, either directly or indirectly. For example, several studies have shown that the Nc distinguishes mother's face from a stranger's face across several ages, even when the two stimuli are presented with equal probability (38, 33, 44, 45, but see also 46). Furthermore, in studies of memory for actions performed with objects in 9- and 10-month old infants, the latency of the Nc was found to be longer for pictures of the familiar action sequences versus unfamiliar action sequences. Critically, the magnitude of the latency difference between the familiar and unfamiliar stimuli predicted behavioral performance in the memory test one month later, suggesting that the Nc was modulated by mnemonic mechanisms (47, 48, 49). The role of mnemonic mechanisms in modulating the Nc component has also received support from research using a cross-modal recognition memory paradigm in typically developing infants (e.g., 50) and those with putatively impaired memory systems (51, 52).
The specific roles of attention versus memory in modulating the Nc component under various circumstances remains a topic of debate (see 53 for discussion). However, the results of a recent source modeling study may provide a basis for resolving this debate in the future. Specifically, these data suggest the possibility that there may actually be two major generators that underlie the Nc component, which differentially index mnemonic and attentional mechanisms. One of the sources identified in this study was a prefrontal source that was active earlier and influenced by stimulus familiarization, and the other was a frontal pole source that was active later and not influenced by stimulus familiarization (54). These findings suggest the distinct possibility that the scalp-recorded Nc may reflect two somewhat distinct processes, which may be more clearly distinguishable from one another with future research.
Two slow wave components, one positive (PSW) and one negative (NSW), have been observed to follow the Nc component (55, 56, 57). These components appear to be more distinctly involved in mnemonic versus attentional processing (53). Specifically, the PSW is believed to reflect the updating of memory representations for partially encoded stimuli, and evidence suggests it may reflect activity in temporal lobe regions involved in memory (54, see also 58). The NSW has been interpreted to reflect the detection of novelty, and evidence suggests it may be generated by regions of the frontal cortex (54). Together, these three components (Nc, PSW, NSW) provide a strong context for the study of memory development in infants and children.
How have ERPs been used to study atypical development?
ERPs have been used extensively to study atypical development or infants/children at risk for falling off a typical developmental trajectory. Below we sample broadly from just two such areas: children experiencing early and varying degrees of psychosocial deprivation; and autism.
Neural Correlates of Emotion Processing in Children Experiencing Early Psychosocial Neglect
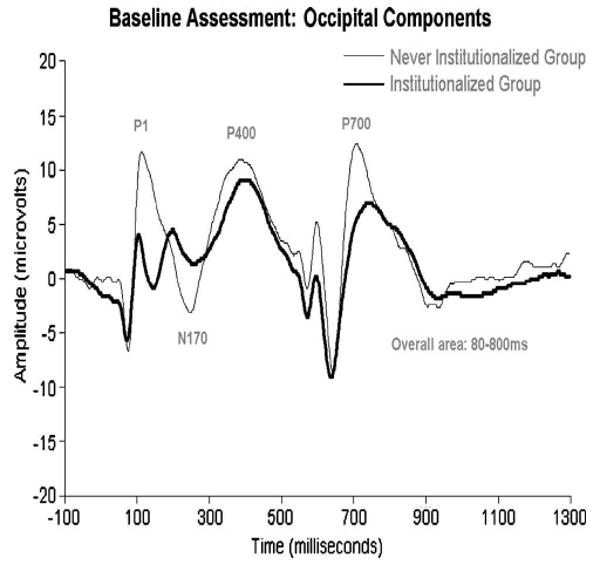
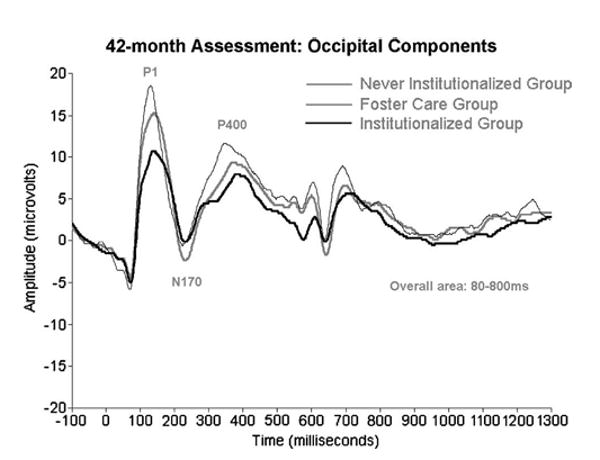
Zeanah, Fox, and Nelson have charted the development of three groups of children in Romania: those abandoned at birth, placed and then raised in institutions; those abandoned at birth, placed in institutions, and then placed in high quality foster care; and those reared since birth with biological families (for details, see 59). Two ERP manipulations were performed. In one, infants/children were presented with alternating images of their caregiver's face and the face of a stranger; in another, they were presented with images of happy, fearful, angry, and sad faces. Across both manipulations, at baseline (prior to randomization to foster care; mean age=22 months) infants in the institutionalized group showed remarkably reduced ERP amplitudes compared to the never institutionalized group (see Figures 1 and 2). Similarly, at 42 months of age, children now living in foster care showed ERP amplitudes that were at the midpoint between the institutionalized and never institutionalized children (see Figures 3 and 4).
Figure 1.
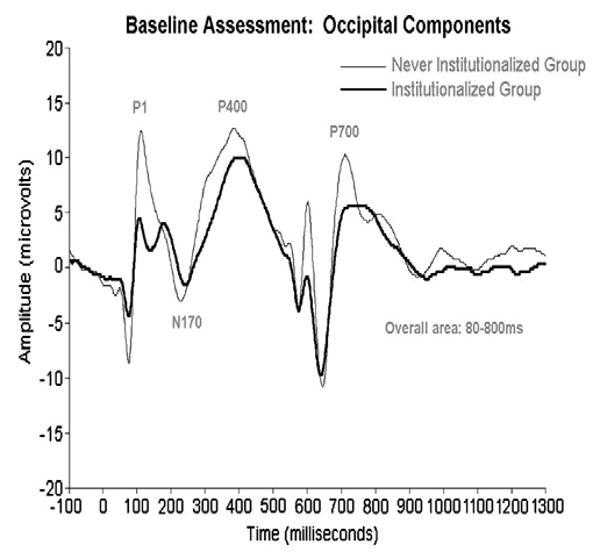
ERPs recorded from institutionalized and never institutionalized infants at baseline (prior to placement in foster care; average age=22 months), to happy, fear, anger, and sad faces. Because there were no group differences in responding to the 4 emotions, the data were collapsed across emotion to highlight the amplitude differences between the groups.
Figure 2.
ERPs recorded from institutionalized children, children living in foster care having previously experienced institutionalization, and never institutionalized children at 42 months of age to happy, fear, anger, and sad faces. As discussed in the text, the children living in foster care show ERP amplitudes that are at the midpoint between institutionalized and never institutionalized children, suggesting improvement over time.
Figure 3.
ERPs recorded from institutionalized and never institutionalized infants at baseline (prior to placement in foster care; average age=22 months), to pictures of the child's caregiver and the face of a stranger. As noted in the text, the groups showed a differential response to these faces; for purposes of this figure, however, we have collapsed across stimulus to reveal the differences in ERP amplitudes across the two groups.
Figure 4.
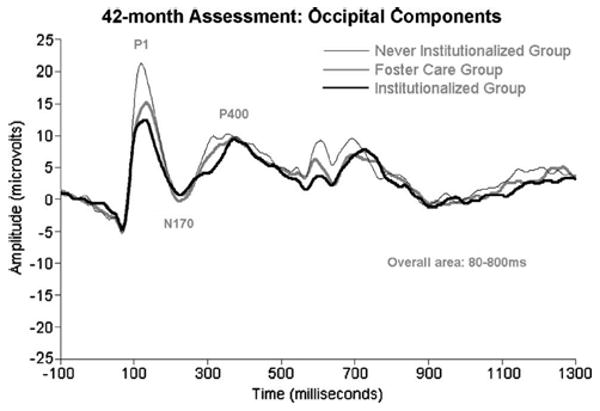
ERPs recorded from institutionalized children, children living in foster care having previously experienced institutionalization, and never institutionalized children at 42 months of age, to pictures of their caregiver's face and the face of a stranger. Again, as with Figure 3, we have collapsed over stimulus to reveal the group differences in ERP amplitudes. As was the case with the facial emotion manipulation (see Figure 2), the ERPs of the foster care group are beginning to normalize.
Over and above reduced ERP amplitudes, two additional observations are worth noting. First, regarding emotion recognition, the institutionalized group performed very similarly to the never institutionalized group; that is, the NC was largest to fearful faces than other faces. Thus, it appears that institutionalization has no effect on discriminating facial expressions of emotion (for elaboration, see 60, 61). Second, there were rather dramatic group differences between institutionalized vs. never institutionalized children, with the former showing larger ERP responses to stranger's faces and the latter showing larger responses to caregiver's faces. Therefore, institutionalization does appear to have an impact on the neural systems involved in facial recognition (for discussion, see Moulson, Westerlund and Nelson, in press, 60, 61).
Overall, these findings illustrate how ERPs have been used to study the neural correlates of different dimensions of face processing among children experiencing early psychosocial deprivation. Similar investigations have been conducted on children experiencing maltreatment, although space limitations prevent us from discussing this work (62, 63, 64, and see also 65).
Autism
Researchers using ERPs have revealed abnormalities in the early stages of face processing in autism (see 66 for review). For example, McPartland and colleagues found that adolescents and adults with autism exhibited slower than normal peak N170 responses to faces but normal latency responses to objects relative to typically developing controls (67). Individuals with autism in this study also failed to show a ‘face inversion effect’ in the N170 component (67). The latter finding suggests a reduced reliance on holistic processing mechanisms for face processing. Further evidence for impaired holistic processing of faces in autism comes from a study showing no difference in EEG power in the gamma band (∼40 Hz) in response to upright versus inverted faces in adults with autism, which was due to reduced gamma power during upright face processing relative to controls (68). This finding suggests reduced neural / perceptual binding in response to the upright faces in the individuals with autism (69, 70). More recent ERP data collected from children with autism spectrum disorders (∼11 years old) showed that these children exhibited reduced source power in frontal regions during the time window of the N170 component during the processing of faces that were filtered to include only low spatial frequency information, but no differences in the processing of faces filtered to include only high spatial frequency information (71). These results also suggest a reduced degree, or reduced depth, of holistic processing in individuals with autism.
In an effort to determine whether the broader autism phenotype is associated with abnormalities in early stage face processing, Dawson and colleagues studied ERPs to upright and inverted faces and objects in parents of children with autism and control participants (72). They found that N170 responses were faster to faces than to objects in the control group, but not in the parents of children with autism. They also found that control participants exhibited right-stronger-than-left responses to the faces but the parents of children with autism did not. These data reflect early stage face processing abnormalities that are consistent with those observed in individuals diagnosed with autism and, therefore, indicate that abnormalities in the neural circuitry involved in early stages of face processing may be a functional trait marker for genetic risk for autism.
Several other studies conducted by Dawson and colleagues have shed light on the nature of face processing impairments in young children with autism (73, 74, 75). In one study, Dawson, Carver, and colleagues examined the neural correlates of familiar and unfamiliar face and object processing in 3- to 4-year old children with autism spectrum disorders (ASD), children with developmental delays (DD), and typically developing children (TD) (74). As expected, the ERPs of TD children differentiated between familiar and unfamiliar faces in two ERP components: The P400 recorded from electrodes over occipital-temporal cortex, and the Nc recorded from frontal and midline electrodes. Unlike these TD children, 3- to 4-year old ASD children did not show differentiation of familiar and unfamiliar faces in either of these ERP components. Like the TD children, however, they did show differential responses to familiar and unfamiliar objects in both the P400 and Nc components. Control children with DD in this study did not show differential P400 or Nc responses to familiar and unfamiliar faces or to familiar and unfamiliar objects. However, they showed differentiation of both in a positive component that followed the Nc, the Positive Slow Wave (PSW), that neither the ASD nor the TD children did. These results suggest that autism is associated with face-specific recognition memory impairment early in life.
In a follow-up study Dawson, Webb, and colleagues examined facial emotion processing in young children with autism (73). In this study, they found that typically developing children exhibited larger amplitude responses in the N290 and NSW components to a face posed in a fearful expression compared with a face posed in a neutral expression, whereas the children with ASD did not. Furthermore, the latency of the N290 component in response to the fear face was associated with better performance on naturalistic experimental assessments of diagnostic social behaviors (i.e., social orienting, joint attention, and attention to distress). These data provide evidence for abnormal processing of facial expressions of emotion at both the perceptual and early cognitive stages of processing, and further suggest that these abnormalities are meaningfully related to impaired social functioning in these children.
In 2006, Webb, Dawson, and colleagues examined early stage face versus object processing in 3- to 4-year old children with autism (75). To do so, they re-analyzed the ERP data collected in the study of familiar and unfamiliar face and object processing (74 described above), collapsing the data across familiarity. Statistical analysis of the face-sensitive N290 component revealed abnormal patterns of face and object processing in the ASD children relative to children in the two control groups. Specifically, comparisons of N290 latencies revealed a significant interaction whereby TD children processed faces faster than objects, but ASD children processed objects faster than faces. Unlike the TD or ASD children, DD children showed similar latency N290 responses to faces and objects. However, the relationship between stimulus type and subject group was different between the ASD children (object latencies shorter than faces) and DD children (equal latency responses to faces and objects). The ASD children also exhibited a reduced amplitude response to the object stimulus relative to both groups of control children. Because effects of familiarity on the N290 were not examined, it is possible that the observed effects were driven by an interaction among familiarity, stimulus type, and the subject groups. However, these data provide preliminary evidence to suggest that early stage face versus object processing is abnormal in young children with autism, and further suggest that these abnormalities may be characterized by differences in both object and face processing in these children relative to controls.
Conclusions
Our goal in writing this paper was to introduce the reader to the utility of recording ERPs in the context of studying both typical development as well as developmental psychopathology. There is a growing literature using this method with a variety of risk and impaired populations, including children with ADHD, children with histories of maltreatment, children experiencing prenatal drug exposure, children suffering from dyslexia and other learning (and memory) problems, and children on the autism spectrum (and those at risk for developing autism). The advantages ERPs hold over other neuroimaging tools include their ease in application, the fact that they can be used across the entire lifespan, their superb temporal resolution and their (relative) inexpense. Their disadvantages include (relatively) poor spatial resolution.
What does the future hold? First, serious efforts are currently being implemented to improve the spatial resolution of ERPs by using higher density arrays of electrodes and in more sophisticated methods of source modeling. Second, a number of laboratories are currently co-registering ERPs with other imaging modalities (e.g., fMRI). We contend that continued refinement of this method, combined with the development of other imaging tools (e.g., Near Infrared Spectroscopy) has the potential to revolutionize our understanding of disorders. When combined with genetic/genomic information, we are optimistic that we are on the threshold of making dramatic breakthroughs in our understanding of mental health problems in children.
Contributor Information
Charles A Nelson, III, Harvard Medical School
Joseph P. McCleery, Harvard Medical School
References
- 1.Johnson MH. Developmental Cognitive Neuroscience (Fundamentals of Cognitive Neuroscience) 2nd. Blackwell Publishing; Oxford, UK: 2005. [Google Scholar]
- 2.Nelson CA, de Haan M, Thomas KM. Neuroscience and Cognitive Development: The Role of Experience and the Developing Brain. New York, NY: John Wiley & Sons; 2006. [Google Scholar]
- 3.de Haan M, Johnson MH, editors. The Cognitive Neuroscience of Development. Psychology Press; 2003. [Google Scholar]
- 4.Nelson CA, Luciana M. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 5.Nelson CA, Luciana M. Handbook of Developmental Cognitive Neuroscience. 2nd. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- 6.Näätänen R, Gaillard AW, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42(4):313–29. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 7.Näätänen R, Alho K. Mismatch negativity--a unique measure of sensory processing in audition. Int J Neurosci. 1995;80(14):317–37. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- 8.Alho K, Woods DL, Algazi A, Näätänen R. Intermodal selective attention. II. Effects of attentional load on processing of auditory and visual stimuli in central space. Electroencephalogr Clin Neurophysiol. 1992;82(5):356–68. doi: 10.1016/0013-4694(92)90005-3. [DOI] [PubMed] [Google Scholar]
- 9.Lyytinen H, Blomberg AP, Näätänen R. Event-related potentials and autonomic responses to a change in unattended auditory stimuli. Psychophysiology. 1992;29(5):523–34. doi: 10.1111/j.1469-8986.1992.tb02025.x. [DOI] [PubMed] [Google Scholar]
- 10.Cowan N, Winkler I, Teder W, Näätänen R. Memory prerequisites of mismatch negativity in the auditory event-related potential (ERP) J Exp Psychol Learn Mem Cogn. 1993;19(4):909–21. doi: 10.1037//0278-7393.19.4.909. [DOI] [PubMed] [Google Scholar]
- 11.Winkler I, Karmos G, Näätänen R. Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain Res. 1996;742(12):239–52. doi: 10.1016/s0006-8993(96)01008-6. [DOI] [PubMed] [Google Scholar]
- 12.Bishop DV. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychol Bull. 2007;133(4):651–72. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- 13.Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipala J, Naatanen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatr Res. 2007;56(2):291–7. doi: 10.1203/01.PDR.0000132750.97066.B9. 4. [DOI] [PubMed] [Google Scholar]
- 14.Gomot M, Bruneau N, Laurent JP, Barthélémy C, Saliba E. Left temporal impairment of auditory information processing in prematurely born 9-year-old children: an electrophysiological study. Int J Psychophysiol. 2007;64(2):123–9. doi: 10.1016/j.ijpsycho.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Korpilahti P, Jansson-Verkasalo E, Mattila ML, et al. Processing of affective speech prosody is impaired in Asperger syndrome. J Autism Dev Disord. 2007;37(8):1539–49. doi: 10.1007/s10803-006-0271-2. [DOI] [PubMed] [Google Scholar]
- 16.Lepistö T, Kajander M, Vanhala R, et al. The perception of invariant speech features in children with autism. Biol Psychol. 2008;77(1):25–31. doi: 10.1016/j.biopsycho.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Thönnessen H, Zvyagintsev M, Harke KC, et al. Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients A combined EEG and MEG study. Biol Psychol. 2008;77(2):205–16. doi: 10.1016/j.biopsycho.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Bentin S, Allision T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cog Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossion B, Gauthier I. How does the brain process upright and inverted faces? Behav Cog Neurosci Rev. 2002;1(1):63–75. doi: 10.1177/1534582302001001004. [DOI] [PubMed] [Google Scholar]
- 20.Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15(8):1261–5. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- 21.Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20(3):1609–24. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Shibata T, Nishijo H, Tamura R, et al. Generators of visual evoked potentials for faces and eyes in the human brain as determined by dipole localization. Brain Topogr. 2002;15(1):51–63. doi: 10.1023/a:1019944607316. [DOI] [PubMed] [Google Scholar]
- 23.Anaki D, Zion-Golumbic E, Bentin S. Electrophysiological neural mechanisms for detection, configural analysis and recognition of faces. Neuroimage. 2007;37(4):1407–16. doi: 10.1016/j.neuroimage.2007.05.054. 1. [DOI] [PubMed] [Google Scholar]
- 24.Flevaris AV, Robertson LC, Bentin S. Using spatial frequency scales for processing face features and face configuration: An ERP analysis. Brain Res. 2008;1194:100–9. doi: 10.1016/j.brainres.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 25.Macchi Cassia V, Kuefner D, Westerlund A, Nelson CA. Modulation of face-sensitive event-related potentials by canonical and distorted human faces: the role of vertical symmetry and up-down featural arrangement. J Cogn Neurosci. 2006;18(8):1343–58. doi: 10.1162/jocn.2006.18.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka JW, Curran T. A neural basis for expert object recognition. Psychol Sci. 2001;12(1):43–7. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- 27.Rossion B, Kung CC, Tarr MJ. Visual expertise with nonface objects leads to competition with the early perceptual processing of faces in the human occipitotemporal cortex. Proc Natl Acad Sci U S A. 2004;101(40):14521–6. doi: 10.1073/pnas.0405613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. J Cogn Neurosci. 2004;16(8):1426–42. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- 29.de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: a review. Int J Psychophysiol. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 30.de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. J Cogn Neurosci. 2002;14(2):199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- 31.Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19(3):1180–93. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 32.Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. J Child Psychol Psychiatry. 2004;45(7):1228–34. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 33.de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Dev Psychol. 1999;35(4):1113–21. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- 34.Leppänen JM, Moulson MC, Vogel-Farley VK, Nelson CA. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007;78(1):232–45. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson CA, de Haan M. Neural correlates of infants' visual responsiveness to facial expressions of emotion. Dev Psychobiol. 1996;29(7):577–95. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 36.de Haan M, Belsky J, Reid V, Volein A, Johnson MH. Maternal personality and infants' neural and visual responsivity to facial expressions of emotion. J Child Psychol Psychiatry. 2004;45(7):1209–18. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 37.Leppänen JM, Nelson CA. The Development and Neural Bases of Recognizing of Facial Emotion. In: Kail R, editor. Advances in Child Development and Behavior. San Diego, CA: Elsevier Press; 2006. pp. 207–246. [DOI] [PubMed] [Google Scholar]
- 38.de Haan M, Nelson CA. Recognition of the mother's face by six-month-old infants: a neurobehavioral study. Child Dev. 1997;68(2):187–210. [PubMed] [Google Scholar]
- 39.Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Dev. 1981;52(3):804–11. [PubMed] [Google Scholar]
- 40.Karrer JH, Karrer R, Bloom D, Chaney L, Davis R. Event-related brain potentials during an extended visual recognition memory task depict delayed development of cerebral inhibitory processes among 6-month-old infants with Down syndrome. Int J Psychophysiol. 1998;29(2):167–200. doi: 10.1016/s0167-8760(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 41.Hunter S, Karrer R. ERPs indicate event duration effects on infants' visual attention and recognition memory. EEG & Clinical Neurophysiology. 1993;87:S38. [Google Scholar]
- 42.Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. New York, NY: Gilford Press; 1994. pp. 269–313. [Google Scholar]
- 43.Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Dev Sci. 2003;6(3):312–28. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier R, Georgieff M. Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci. 2000;114(5):950–6. [PubMed] [Google Scholar]
- 45.Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Dev Sci. 2005;8(6):605–16. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- 46.Carver LJ, Dawson G, Panagiotides H, et al. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Dev Psychobiol. 2003;42(2):148–59. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer PJ, Wiebe SA, Carver LJ, et al. Electrophysiological indexes of encoding and behavioral indexes of recall: examining relations and developmental change late in the first year of life. Dev Neuropsychol. 2006;29(2):293–320. doi: 10.1207/s15326942dn2902_2. [DOI] [PubMed] [Google Scholar]
- 48.Bauer PJ, Wiebe SA, Carver LJ, Waters JM, Nelson CA. Developments in long-term explicit memory late in the first year of life: behavioral and electrophysiological indices. Psychol Sci. 2003;14(6):629–35. doi: 10.1046/j.0956-7976.2003.psci_1476.x. [DOI] [PubMed] [Google Scholar]
- 49.Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Dev Sci. 2000;3:234–246. [Google Scholar]
- 50.Nelson CA, Henschel M, Collins PF. Neural correlates of cross-modal recognition memory by 8-month-old infants. Dev Psychol. 1993;29:411–42. [Google Scholar]
- 51.deRegnier RA, Long JD, Georgieff MK, Nelson CA. Using event-related potentials to study perinatal nutrition and brain development in infants of diabetic mothers. Dev Neuropsychol. 2007;31(3):379–96. doi: 10.1080/87565640701229524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson CA, Wewerka SS, Borscheid AJ, Deregnier RA, Georgieff MK. Electrophysiologic evidence of impaired cross-modal recognition memory in 8-month-old infants of diabetic mothers. J Pediatr. 2003;142(5):575–82. doi: 10.1067/mpd.2003.210. [DOI] [PubMed] [Google Scholar]
- 53.de Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and Event-Related Potentials: Studies in Developmental Psychology. New York, NY: Psychology Press; 2006. [Google Scholar]
- 54.Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source localization study. Dev Psychol. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson CA, Collins PF. Event-related potentials and looking-time analysis of infants' responses to familiar and novel events: Implications for recognition memory. Dev Psychol. 1991;27:50–58. [Google Scholar]
- 56.Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain Cogn. 1992;19(1):105–21. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- 57.Nelson CA, deRegier RA. Neural correlates of attention and memory in the first year of life. Dev Neuropsychol. 1992;8:119–134. [Google Scholar]
- 58.Nelson CA. Electrophysiological correlates of memory development in the first year of life. In: Reese H, Franzen M, editors. Biological and neuropsychological mechanisms: Life span developmental psychology. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1996. pp. 95–131. [Google Scholar]
- 59.Zeanah CH, Nelson CA, Fox NA, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev Psychopath. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- 60.Parker SW, Nelson CA, et al. The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: an event-related potential study. Child Dev. 2005;a76(1):54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 61.Parker SW, Nelson CA. the BEIP Core Group An event-related potential study of the impact of institutional rearing on face recognition. Dev Psychopathol. 2005;b17:621–639. doi: 10.1017/S0954579405050303. [DOI] [PubMed] [Google Scholar]
- 62.Cicchetti D, Curtis WJ. An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Dev Psychopathol. 2005;17:641–677. doi: 10.1017/S0954579405050315. [DOI] [PubMed] [Google Scholar]
- 63.Pollak SD, Cicchetti D, Klorman R, Brumaghim JT. Cognitive brain event-related potentials and emotion processing in maltreated children. Child Dev. 1997;68(5):773–787. doi: 10.1111/j.1467-8624.1997.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 64.Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children's reactions to facial displays of emotion. Psychophysiology. 2001;38:267–274. [PubMed] [Google Scholar]
- 65.Curtis WJ, Cicchetti D. Emotion and resilience: A multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Dev Psychopathology. 2007;19:811–840. doi: 10.1017/S0954579407000405. [DOI] [PubMed] [Google Scholar]
- 66.Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27(3):403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 67.McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiatry. 2004;45(7):1235–45. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 68.Grice SJ, Spratling MW, Karmiloff-Smith A, et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12(12):2697–700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- 69.Herrmann CS, Mecklinger A, Pfeifer E. Gamma responses and ERPs in a visual classification task. lin Neurophysiol. 1999;110(4):636–42. doi: 10.1016/s1388-2457(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 70.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16(13):4240–9. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boeschoten MA, Kenemans JL, van Engeland H, Kemner C. Face processing in Pervasive Developmental Disorder (PDD): the roles of expertise and spatial frequency. J Neural Transm. 2007;114(12):1619–29. doi: 10.1007/s00702-007-0780-y. [DOI] [PubMed] [Google Scholar]
- 72.Dawson G, Webb SJ, Wijsman E, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol. 2005;17(3):679–97. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- 73.Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Dev Sci. 2004;7(3):340–59. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 74.Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700–17. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. J Autism Dev Disord. 2006;36(7):881–90. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


