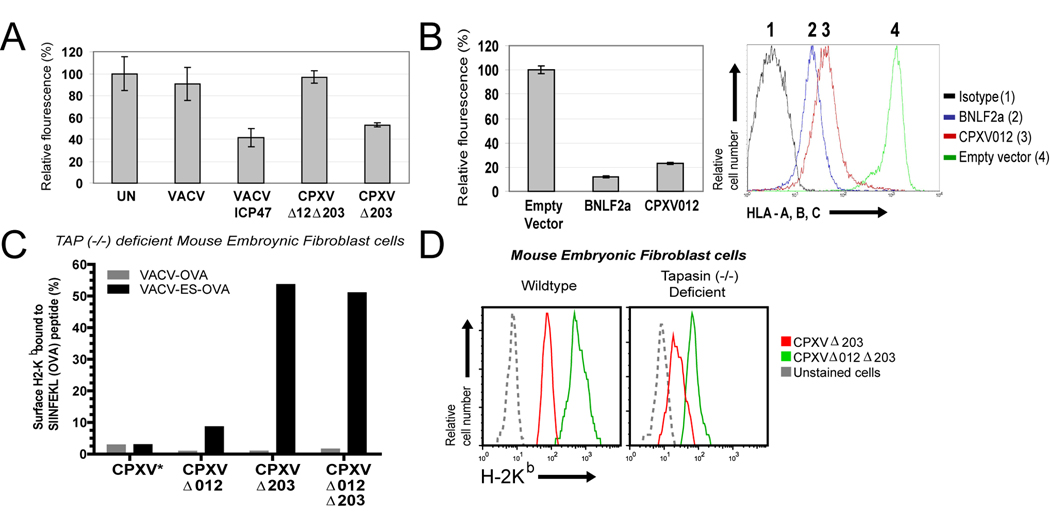
Figure 7. CPXV012 inhibits TAP-dependent peptide transport.
(A) Peptide transport in the presence of CPXV012. HeLa cells were infected with indicated viruses and at 6 hpi permeabilized with SLO followed by incubation with peptide FITC~CVNKTERAY in the presence or absence of ATP in transport buffer. Recovered fluorescence was background (−ATP) adjusted and is shown as percent relative to uninfected cells. (B) Peptide transport in CPXV012-expressing cells. Peptide transport was measured in MJS cells stably transduced with GFP-expressing control lentiviruses or lentiviruses expressing either BNLF2a or CPXV012 (left panel). Transduction efficiency was verified by monitoring MHC-I surface levels by flow cytometry (right panel). CPXV012, BNLF2a, GFP control, and isotype control are represented by red, blue, green, and black lines, respectively. (C) CPXV012 does not inhibit TAP-independent peptide loading. TAP−/− MEFs were infected with VACV-OVA or VACV-ES-OVA together with indicated CPXV recombinants (MOI=10 each) and simultaneously treated with 25 U/ml IFN-γ. At 24 hpi, SIINFEKL/H2-Kb complexes were measured with specific Ab 25.D1.16 by flow cytometry. The percentage of GFP+ cells that were also 25.D1.16 positive is shown except for WT-CPXV showing total 25.D1.16-positive cells (*). (D) MHC-I downregulation by CPXV012 does not require Tapasin. WT and TPN−/− MEFs were infected with indicated viruses (MOI=5) in the presence of IFN-γ and surface H2-Kb was monitored by flow cytometry at 24 hpi. Kb-expression is shown for GFP+ cells.