Abstract
We tested the hypothesis that the comorbidity of migraine and epilepsy results from a shared genetic susceptibility to the two disorders. We used semistructured telephone interviews to collect information on migraine and epilepsy in the families (parents, siblings, and offspring) of 1,957 adult probands with epilepsy. Epilepsy was defined as a lifetime history of two or more unprovoked seizures, and migraine as self-reported severe headaches with two or more of the following symptoms: unilateral pain, throbbing pain, visual aura, or nausea. As a first test of the hypothesis of shared susceptibility, we assessed risk of migraine in relatives of probands with genetic versus nongenetic forms of epilepsy, using two proxy measures of genetic susceptibility—a first-degree family history of epilepsy and idiopathic/cryptogenic (versus postnatal symptomatic) etiology. Neither of these two measures was associated with risk of migraine in relatives. As a second test, we assessed risk of epilepsy in the relatives of probands with versus without migraine. With the exception of one subgroup (sons of female probands), risk of epilepsy in relatives was not associated with the proband's history of migraine. This pattern of results is inconsistent with the hypothesis of a shared genetic susceptibility to migraine and epilepsy.
Migraine is an extremely common disorder, with a prevalence of almost 18% in females and 6% in males.1 Though less common than migraine, epilepsy is also one of the most common neurologic disorders, with a prevalence of 0.5%.2 Moreover, the two disorders appear to be associated.3,4 In a recent study, we found that the risk of migraine was more than twice as high in persons with epilepsy as in those without epilepsy.4 The increased risk of migraine was evident within every subgroup of epilepsy defined by seizure type, age at onset, etiology, or family history of epilepsy in first-degree relatives. Age-specific incidence of migraine in subjects with epilepsy was increased to a greater extent after onset of epilepsy than before, but was also significantly increased >5 years before onset and 1 to 5 years before onset.
The causes of the comorbidity of migraine and epilepsy are unknown. A small part of the comorbidity appears to result from an effect of head injury on risk of both disorders. Head injury is a known risk factor for both migraine5 and epilepsy,6 and risk of migraine was significantly higher in subjects with post-traumatic epilepsy than in subjects with other etiologies of epilepsy. However, the association cannot be completely explained by identified shared environmental risk factors because risk of migraine was significantly increased in persons with idiopathic/cryptogenic epilepsy, where by definition environmental risk factors are absent.
There is strong evidence for a genetic influence on susceptibility to epilepsy.7 Genetic influences are also suggested in migraine,8 and clearly play a role in certain migraine subtypes.9 Thus one possible explanation for comorbidity of the two disorders is that there is a shared genetic susceptibility to migraine and epilepsy.
If there are shared genetic contributions to migraine and epilepsy, we would predict (1) a greater risk of migraine in the families of subjects likely to have genetic versus nongenetic forms of epilepsy and (2) a greater risk of epilepsy in the families of epilepsy patients with versus without migraine. In this paper, we explore the possibility of a shared genetic susceptibility by testing these predictions using data from the Epilepsy Family Study of Columbia University, our ongoing study of genetic influences on epilepsy and related disorders.
Methods
Data collection
The methods for data collection in the Epilepsy Family Study of Columbia University have been described in detail previously.10 Briefly, we ascertained 1,957 subjects with epilepsy who were aged ≥18 years (probands) from 10 voluntary organizations for epilepsy through a telephone survey conducted between 1985 and 1988. We used semistructured telephone interviews with the probands to collect personal and family history data on seizures and other disorders in parents, full and half-siblings, offspring, and spouses. We reviewed medical records for 60% of the probands.
We also administered semistructured interviews to 1,423 parents and full siblings of the probands (949 mothers, 98 fathers, 241 sisters, 135 brothers). These relatives were interviewed for two reasons. First, we attempted to interview a second informant in each family to increase accuracy and completeness of the family history information. We selected the mother as the second informant whenever possible; if she was unavailable, we gave second priority to the eldest sister. This protocol accounts for the excess of mothers and sisters among interviewed relatives. Second, we attempted to interview each adult relative (≥18 years) reported to have had seizures after age 5 years in order to confirm and augment the seizure history.
The participation rate for probands was 84%. Eighty-seven percent of probands were white, and 60% were women. We did not screen probands formally for intelligence, but interviewers judged them to be capable of understanding and answering the interview questions. Eighty-seven percent were high school graduates, and 55% had ≥1 year of college education. The average age of the probands was 36 ± 11 (SD) years, that of the interviewed parents was 60 ± 9 (SD), and that of the interviewed siblings was 42 ± 12 (SD).
Case definitions
The diagnosis of epilepsy in both probands and relatives was based on a case-by-case review of all available information (proband interview, second informant interview, direct interview, and medical record). Epilepsy was defined as a lifetime history of two or more unprovoked seizures,2 and was subclassified by seizure type, age at onset, and presumed etiology according to standardized criteria.2 Seizures were classified according to the 1981 criteria of the International League Against Epilepsy.11 As we have demonstrated previously, the resulting seizure classifications were reliable (reproducible).12 Furthermore, seizure classifications based on interview data alone proved to be valid, using the diagnoses of expert physicians as the gold standard.13 Thus diagnostic accuracy was probably not compromised by the low proportion (60%) of probands for whom medical records were available.
We used three categories of etiology in probands—idiopathic/cryptogenic: epilepsy occurring in the absence of an historical insult to the CNS demonstrated to greatly increase the risk of unprovoked seizures; neurologic deficit presumed present at birth (neurodeficit from birth): epilepsy associated with a history of cerebral palsy (motor handicap or movement disorder) or mental retardation (IQ <70) presumed present at birth; and postnatal symptomatic: epilepsy associated with a history of a postnatal CNS insult occurring ≥7 days prior to the first unprovoked seizure.14,15
Migraine headache was defined as a self-reported history of severe headaches with two or more of the following symptoms: unilateral pain, throbbing pain, visual aura (specifically flashing lights, spots before eyes, or both), and nausea. This case definition differs somewhat from that of the International Headache Society (IHS) because we began data collection prior to publication of the IHS criteria.16 In subjects classified as having migraine, we defined the age at onset of migraine as the age at reported occurrence of the first severe headache.
Statistical analysis
We performed two separate analyses to evaluate risks of migraine and epilepsy in the relatives of probands with epilepsy. In the first set of analyses, we tested the hypothesis that risk of migraine was higher in the families of probands with genetic versus nongenetic forms of epilepsy. For this purpose, we subdivided the probands by their likelihood of having a genetic susceptibility to epilepsy and then compared risks of migraine in the families of probands in these different groups. We used two variables as proxy measures of the likelihood of a genetic susceptibility. First, we assumed that probands with a positive family history of epilepsy (defined as one or more affected first-degree relatives) were more likely to have a genetic susceptibility than those with a negative family history. Second, we assumed that probands with idiopathic/cryptogenic epilepsy were more likely to have a genetic susceptibility than those with remote symptomatic epilepsy. Previous studies have indicated higher risks of epilepsy in relatives of probands with idiopathic/cryptogenic epilepsy than in relatives of those with symptomatic epilepsy.7 In our own previous investigations of this data-set,14,15 risk of epilepsy was not increased in relatives of probands with postnatal symptomatic epilepsy. However, risk was increased in relatives of probands with neurodeficits. Because of this difference, we assessed the risk of migraine in relatives of probands in these two subgroups separately.
Although probands were asked about migraine headaches in their relatives, the sensitivity of this family history data was only 44% compared with diagnoses based on direct interview.17 Consequently, the analysis of migraine risk was restricted to relatives interviewed directly. We classified subjects who responded “don't know” to the question about severe headaches (six probands, zero relatives) as unaffected with migraine on the assumption that migraine headaches would have been severe enough to preclude a “don't know” answer. We excluded subjects who reported “yes” to severe headaches if they did not meet criteria for migraine, but reported “don't know” to two or more of the symptoms required for diagnosis (nine probands, five relatives). Among the 1,423 relatives with direct interviews, we excluded those who were classified as unknown with respect to either migraine (N = 5) or epilepsy (N = 7) and those of probands with unknown migraine status (N = 6). The remaining 1,405 relatives were included in analyses of migraine in relatives.
In the second set of analyses, we tested the hypothesis that risks of epilepsy were higher in relatives of probands with migraine than in relatives of probands without migraine. For analyses of epilepsy risks in relatives, we included all relatives regardless of whether or not they had been interviewed directly. The proband's family history report of epilepsy in parents and siblings had excellent validity (sensitivity 87%, specificity 99%), using the mother's report as the gold standard.18 Furthermore, restriction of the analyses to relatives who had been interviewed directly would have introduced a significant bias because our data collection protocol involved selection of relatives for interview if they were suspected of having epilepsy.10
We used survival analysis methods19,20 to control for differences in years-at-risk of migraine and epilepsy between different comparison groups. For this purpose, we assumed that each subject was at risk of developing migraine or epilepsy from birth until current age or age at death (if unaffected), or age at onset of the disorder. We used actuarial life table analysis19 to compute age-specific cumulative incidence of epilepsy and migraine, and univariate and multivariate Cox proportional hazards analysis20 to compute rate ratios (RRs) for each disorder according to various proband characteristics.
Results
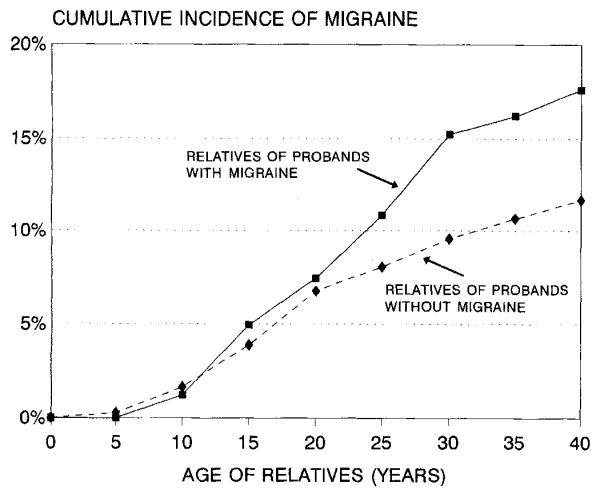
Risk of migraine in the relatives was associated with three factors that were potential confounders in our analysis. First, migraine was familial, with a 1.5-fold increased risk in relatives of probands with migraine compared with relatives of probands without migraine (table 1 and figure 1). Second, risk of migraine was higher in relatives with epilepsy than in relatives without epilepsy (RR = 2.3, see table 1), reflecting the comorbidity of epilepsy and migraine that we reported previously.4 Third, risk was higher in female than in male relatives (RR = 1.6, see table 1), reflecting the well-known female preponderance of migraine.1
Table 1.
Rate ratios for migraine in relatives of probands with epilepsy
| No. of relatives |
Rate ratio (95% CI) |
|||
|---|---|---|---|---|
| Characteristics of probands and relatives |
Total | With migraine (%) | Univariate | Multivariate* |
| Family history of epilepsy in addition to proband† | ||||
| Positive | 160 | 30 (18.5) | 1.3 (0.86–1.86) | 1.1 (0.75–1.66) |
| Negative | 1,245 | 191 (15.3) | 1.0 (reference) | 1.0 (reference) |
| Etiology of proband's epilepsy | ||||
| Idiopathic/cryptogenic | 1,136 | 182 (16.0) | 1.0 (0.72–1.43) | 1.0 (0.71–1.43) |
| Neurodeficit | 19 | 0 (—) | — | — |
| Postnatal symptomatic | 250 | 39 (15.6) | 1.0 (reference) | 1.0 (reference) |
| Proband's history of migraine | ||||
| Positive | 323 | 67 (20.7) | 1.5 (1.13–2.01) | 1.5 (1.16–2.07) |
| Negative | 1,082 | 154 (14.2) | 1.0 (reference) | 1.0 (reference) |
| Relative's history of epilepsy | ||||
| Positive | 87 | 23 (26.4) | 2.2 (1.42–3.37) | 2.3 (1.48–3.64) |
| Negative | 1,318 | 198 (15.0) | 1.0 (reference) | 1.0 (reference) |
| Gender of the relative | ||||
| Female | 1,174 | 195 (16.6) | 1.4 (0.93–2.11) | 1.6 (1.04–2.39) |
| Male | 231 | 26 (11.3) | 1.0 (reference) | 1.0 (reference) |
Multivariate analysis includes all variables listed in the table.
Family history of epilepsy in addition to the proband: one or more parents, siblings, or offspring of proband affected with epilepsy, other than the relative whose risk of migraine is being evaluated.
CI = confidence interval.
Figure 1.
Cumulative incidence of migraine in relatives of probands with epilepsy by history of migraine in the proband.
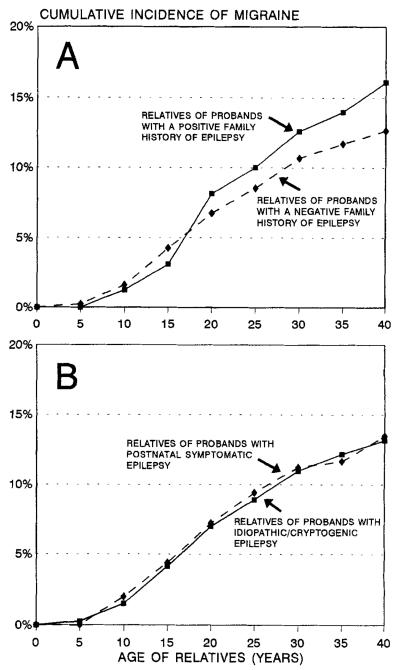
Neither of the two measures of genetic susceptibility to epilepsy in probands was associated with risk of migraine in relatives, however (see table 1 and figure 2). After adjustment for the three potential confounders, the RR for relatives of probands with versus without a family history of epilepsy was 1.1, and that for relatives of probands with idiopathic/cryptogenic versus postnatal symptomatic epilepsy was 1.0 (see table 1). None of the 19 relatives of probands with neurodeficits was affected with migraine.
Figure 2.
Cumulative incidence of migraine in relatives of probands with epilepsy by family history of epilepsy, in addition to the proband (A), and etiology of epilepsy in the proband (B).
Next, we examined the risk of epilepsy in relatives of probands with versus without migraine (table 2). Risk of epilepsy was not significantly associated with the proband's history of migraine in parents or siblings, regardless of the gender of the proband, or in offspring of male probands. However, risk of epilepsy was significantly higher in offspring of female probands with migraine than in offspring of female probands without migraine (RR = 1.8, see table 2). These findings were not affected by adjustment for other factors associated with epilepsy risk in relatives (the proband's age at onset, seizure type, and etiology of epilepsy).
Table 2.
Rate ratios for epilepsy in relatives of probands with epilepsy by history of migraine in the proband
| No. of relatives |
Rate ratio (95% CI) |
|||
|---|---|---|---|---|
| Class of relatives and proband's history of migraine |
Total | With epilepsy (%) | Univariate | Multivariate* |
| Relatives of male probands | ||||
| Parents of: | ||||
| Probands with migraine | 217 | 7 (3.2) | 1.5 (0.65–3.47) | 1.5 (0.65–3.53) |
| Probands without migraine | 1,201 | 26 (2.2) | 1.0 (reference) | 1.0 (reference) |
| Siblings of: | ||||
| Probands with migraine | 333 | 5 (1.5) | 0.6 (0.25–1.61) | 0.7 (0.27–1.76) |
| Probands without migraine | 1,658 | 39 (2.4) | 1.0 (reference) | 1.0 (reference) |
| Offspring of: | ||||
| Probands with migraine | 108 | 2 (1.9) | 1.3 (0.26–5.92) | 1.4 (0.30–6.90) |
| Probands without migraine | 463 | 8 (1.7) | 1.0 (reference) | 1.0 (reference) |
| Relatives of female probands | ||||
| Parents of: | ||||
| Probands with migraine | 581 | 16 (2.8) | 1.4 (0.78–2.66) | 1.5 (0.81–2.75) |
| Probands without migraine | 1,516 | 29 (1.9) | 1.0 (reference) | 1.0 (reference) |
| Siblings of: | ||||
| Probands with migraine | 761 | 22 (2.9) | 1.1 (0.64–1.72) | 1.1 (0.69–1.85) |
| Probands without migraine | 1,986 | 55 (2.8) | 1.0 (reference) | 1.0 (reference) |
| Offspring of: | ||||
| Probands with migraine | 371 | 27 (7.3) | 1.8 (1.06–2.91) | 1.8 (1.09–3.02) |
| Probands without migraine | 776 | 34 (4.4) | 1.0 (reference) | 1.0 (reference) |
Multivariate analysis includes proband's age at onset (0–9, 10–19, ≥20 years), seizure type (generalized onset vs. partial onset), etiology of epilepsy (idiopathic/cryptogenic, neurodeficit, postnatal symptomatic).
CI = confidence interval.
In the offspring of female probands, the association between risk of epilepsy and the proband's history of migraine was observed only in their sons. Cumulative incidence of epilepsy to age 25 was 12% in sons of female probands with migraine and 6% in each of the other three groups of offspring of female probands (sons of probands without migraine and daughters of probands with and without migraine). In contrast, in the offspring of male probands, cumulative incidence of epilepsy to age 25 was 3% and did not differ significantly according to either gender of the offspring or the proband's history of migraine.
In analyses of the relations between the proband's history of migraine and the relatives' histories of epilepsy, migraine in the relatives is a potential confounder. The potential confounding effect occurs because migraine in the relatives is associated with both the proband's history of migraine (due to the familial aggregation of migraine) and the relative's history of epilepsy (due to the comorbidity of epilepsy and migraine). In most of our analyses of epilepsy risk, we did not control for this potential confounding effect because migraine status could be assessed only in relatives who were directly interviewed, and we had selected relatives for interview based on the proband's report of seizure disorders. Because of this selection, restriction of the analysis to relatives interviewed directly would have introduced a bias to the analysis of epilepsy risk in most classes of relatives. This problem does not apply to the mothers of the probands, however, because they were interviewed as second informants about the family medical history, regardless of their own seizure histories. Thus we repeated the analysis of epilepsy risk, restricting it to the mothers who had been interviewed directly. As expected, risk of epilepsy in the mothers was associated with the mothers' histories of migraine, although this association was not statistically significant (RR = 2.3, 95% confidence interval [CI] 0.87–5.85). However, the mothers' risk of epilepsy was not associated with migraine in the proband, either before (RR = 0.6, 95% CI 0.16–1.88) or after adjustment for the mothers' histories of migraine (RR = 0.5, 95% CI 0.15–1.73).
Discussion
We proposed to test the hypothesis that shared genetic susceptibility accounts for the comorbidity of migraine and epilepsy. As a first test of this hypothesis, we compared risks of migraine in relatives of probands with genetic versus nongenetic forms of epilepsy using two proxy measures of genetic susceptibility—a first-degree family history of epilepsy and idiopathic/cryptogenic (versus postnatal symptomatic) epilepsy. Neither of these two measures of genetic susceptibility to epilepsy in probands was associated with risk of migraine in relatives. These findings are consistent with those of a study by Kraus,21 reported by Andermann and Ander-mann,22 in which probands with epilepsy did not differ from controls in terms of the prevalence of migraine in their relatives. These results are not compatible with the hypothesis that shared genetic susceptibility accounts for the comorbidity of migraine and epilepsy.
In assessing risk for migraine in the relatives of probands with epilepsy, we controlled for three factors known or suspected to be associated with migraine risk—the proband's history of migraine, the relative's history of epilepsy, and the relative's gender. Migraine risk was familial, with a significantly higher risk in relatives of probands with migraine than in relatives of those without migraine. The RR of 1.5 is compatible with the findings of previous population-based studies23,24 in which migraine risks were assessed by direct interview with the relatives. Previous studies that relied on proband reports of migraine in relatives or used clinic-based ascertainment schemes may have overestimated the familial aggregation of migraine. Subjects with migraine report migraine in their relatives with greater sensitivity than do controls,17 leading to bias in estimates of relative risk.25 Persons with migraine who seek medical care for their attacks have unusually high migraine disability.26,27 If disability should be associated with familial risk, then ascertainment from clinical settings would also lead to overestimation of relative risk.
As a second test of the hypothesis of shared genetic susceptibility, we assessed risk of epilepsy in relatives of probands with versus without migraine. Migraine in the proband was not associated with epilepsy in either the parents or siblings. There was no association, either, in analyses restricted to the interviewed mothers of the probands, in whom it was possible to control for comorbid migraine in assessing risk of epilepsy. We found an association with epilepsy in only one subgroup of relatives—male offspring of female probands with epilepsy.
This overall pattern of results is inconsistent with predictions based on a model of shared genetic susceptibility to migraine and epilepsy. The single exception occurs in the analysis of sons of female probands with and without migraine. We considered several genetic models that might explain this finding. For each of these genetic models, we postulated that a hypothetical susceptibility genotype increases the risk of both epilepsy and migraine.
First, we considered mitochondrial inheritance. If a susceptibility gene for migraine and epilepsy were located in the mitochondrial genome, we would expect an increased risk of epilepsy in the offspring of female but not male probands with both epilepsy and migraine, as is observed. However, under this model, female probands would be expected to transmit susceptibility with equal probability to their sons and daughters, and not only to their sons. Also, we would expect an increased risk of epilepsy in the mothers of both male and female probands with migraine, which we did not observe. Thus our data are inconsistent with this model.
We can also reject models involving X-linkage. Under an X-linked dominant model, we would expect that risks of epilepsy would be the same in sons and daughters of female probands with both migraine and epilepsy. Under an X-linked recessive model, we would expect higher risks in sons than in daughters of female probands, but we would also expect an increased risk in siblings of female probands with versus without migraine, which we did not observe.
We also considered nongenetic models that might explain this finding. Intrauterine exposures or pregnancy complications would be expected to affect risk in offspring of women but not men with both migraine and epilepsy. However, the relevant exposures would have to be more common in women with both disorders than in women with epilepsy alone, and their effects would have to be restricted to the sons of affected women.
This unpredicted result may have arisen by chance alone. Though statistically significant, the effect is modest. We made multiple comparisons in analyses of epilepsy risk, stratified by the gender of probands and relatives as well as relationship to the proband. The result is inconsistent with the overall pattern of results and is difficult to explain on the basis of any plausible biological model.
The findings in table 2 indicate that risk of epilepsy is higher in offspring of female probands than in offspring of male probands. This maternal effect has been consistently observed in previous studies and cannot be explained by any conventional genetic model.28,29 Our previous analyses indicate that it cannot be explained, either, by intrauterine exposure to seizures or anticonvulsants in offspring of women with epilepsy or by a higher proportion of affected mothers than affected fathers with clinical features of epilepsy associated with high familial risk.30 Perinatal complications that occur with increased frequency in women with epilepsy could not explain the maternal effect either, because they are not associated with increased risk of epilepsy in offspring without cerebral palsy.31
Given our present observation of an association between migraine in female probands and epilepsy risk in their sons, we considered the possibility that the maternal effect might be attributed to a subset of female probands with both epilepsy and migraine. Our data are inconsistent with this possibility, however, because risk was higher in offspring of females than in those of males both for probands with migraine (7.3% versus 1.9%) and for probands without migraine (4.4% versus 1.7%) (table 2).
This study is limited by a number of factors. First, our definition of migraine differs from that recommended by the IHS. Because we began data collection prior to publication of the IHS criteria, we did not collect data on attack duration or frequency, photophobia, or phonophobia. Our criteria would fail to detect rare subtypes of migraine with sensory or hemiplegic aura. On the other hand, we may have included some individuals who do not meet the IHS criteria pertaining to attack duration and frequency. Rates of misclassification caused by these differences in definition would have been nondifferential between our comparison groups and thus could have slightly biased our estimates of RR toward the null hypothesis. However, the degree of misclassification was probably too low to attenuate a strong association between migraine risk in relatives and the proband's family history or etiology of epilepsy.
Second, probands in our series (adults with epilepsy who contacted voluntary organizations for epilepsy) are unrepresentative of the general population of persons with epilepsy. The proportion with partial onset seizures (84%) is higher than in prevalent cases of all ages in Rochester (59%),2 but is similar to that in other series of adults with epilepsy ascertained from clinical care settings.14,15 Furthermore, because our probands are adult prevalent epilepsy cases, persons with childhood-onset epilepsies that remit before adulthood, many of which are associated with high familial risk, were largely excluded from our sample of probands. This selection limits the generalizability of our findings. Thus the genetic relations between epilepsy and migraine could prove to be different in early-onset, remitting epilepsies that were excluded from our series.
Third, although epilepsy status was ascertained for most individuals in every pedigree, migraine status was ascertained only in persons who were interviewed directly. Consequently, when we evaluated the effect of the proband's history of migraine on epilepsy risk in relatives, we could not control for the confounding effect of migraine in the relatives. This confounding effect would be expected to lead to an increased risk of epilepsy in relatives of probands with migraine because (1) migraine in the proband is associated with migraine in relatives (caused by the familial aggregation of migraine) and (2) epilepsy in the relatives is associated with migraine in the relatives (caused by the comorbidity of epilepsy and migraine). This confounding may have partly explained the increased risk of epilepsy we observed in sons of female probands with migraine, although if so, it is difficult to explain why the association was limited to this subgroup.
Despite these limitations, the study results suggest that the comorbidity of migraine and epilepsy cannot be explained by genetic mechanisms which predispose to both disorders. The pattern of results is compatible with the model that both migraine and epilepsy are caused by a condition of neuronal excitability that results from genetic as well as environmental risk factors.
Acknowledgments
Supported by NIH grant RO1-NS20656 and a research grant from Abbott Pharmaceuticals.
Contributor Information
Ruth Ottman, G.H. Sergievsky Center and Epidemiology Division, School of Public Health, Columbia University, and Epidemiology of Brain Disorders Department, New York State Psychiatric Institute, New York.
Richard B. Lipton, Departments of Neurology, and Epidemiology and Social Medicine, Albert Einstein College of Medicine, and Montefiore Headache Unit, Montefiore Medical Center, New York, NY..
References
- 1.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- 2.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 3.Andermann E, Andermann F. Migraine-epilepsy relationships: epidemiological and genetic aspects. In: Andermann FA, Lugaresi E, editors. Migraine and epilepsy. Butter-worth; Boston: 1987. pp. 281–291. [Google Scholar]
- 4.Ottman R, Lipton RB. Comorbidity of migraine and epilepsy. Neurology. 1994;44:2105–2110. doi: 10.1212/wnl.44.11.2105. [DOI] [PubMed] [Google Scholar]
- 5.Shechter A, Stewart W, Celentano D, Linet M. An epidemiologic study of migraine and head injury [abstract] Neurology. 1990;40(suppl 1):345. [Google Scholar]
- 6.Annegers JF, Grabow JD, Groover RV, et al. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- 7.Hauser WA, Annegers JF, Anderson VE. Epidemiology and the genetics of epilepsy. Epidemiology and the genetics of epilepsy. In: Ward AA, Penry JK, Purpura D, editors. Raven Press; New York: 1983. pp. 267–294. [PubMed] [Google Scholar]
- 8.Merikangas KR. Genetic epidemiology of migraine. In: Sandler M, Collins GM, editors. Migraine: a spectrum of ideas. Oxford University Press; New York: 1990. pp. 40–49. [Google Scholar]
- 9.Joutel A, Bousser M-G, Biousse V, et al. A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet. 1993;5:40–45. doi: 10.1038/ng0993-40. [DOI] [PubMed] [Google Scholar]
- 10.Ottman R, Susser M. Data collection strategies in genetic epidemiology: the Epilepsy Family Study of Columbia University. J Clin Epidemiol. 1992;45:721–727. doi: 10.1016/0895-4356(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 11.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 12.Ottman R, Lee JH, Hauser WA, et al. Reliability of seizure classification using a semistructured interview. Neurology. 1993;43:2526–2530. doi: 10.1212/wnl.43.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians' diagnoses. Epilepsia. 1990;31:110–115. doi: 10.1111/j.1528-1157.1990.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottman R, Lee JH, Risch N, Hauser WA, Susser M. Clinical indicators of genetic susceptibility to epilepsy. Epilepsia. 1996;37:353–361. doi: 10.1111/j.1528-1157.1996.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 15.Ottman R, Annegers JF, Risch N, Hauser WA, Susser M. Relations of genetic and environmental factors in the etiology of epilepsy. Ann Neurol. 1996;39:442–449. doi: 10.1002/ana.410390406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(suppl 7):1–96. [PubMed] [Google Scholar]
- 17.Ottman R, Hong S, Lipton RB. Validity of family history data on severe headache and migraine. Neurology. 1993;43:1954–1960. doi: 10.1212/wnl.43.10.1954. [DOI] [PubMed] [Google Scholar]
- 18.Ottman R, Hauser WA, Susser M. Validity of family history data on seizure disorders. Epilepsia. 1993;34:469–475. doi: 10.1111/j.1528-1157.1993.tb02587.x. [DOI] [PubMed] [Google Scholar]
- 19.Cutler SJ, Ederer F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958;8:699–712. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 21.Kraus D. Migraine and epilepsy: a case for divorce. McGill University; Montreal: 1978. Thesis. [Google Scholar]
- 22.Andermann E, Andermann F. Migraine-epilepsy relationships: epidemiological and genetic aspects. In: Andermann FA, Lugaresi E, editors. Migraine and epilepsy. Butter-worths; Boston: 1987. pp. 281–291. [Google Scholar]
- 23.Waters WE. Migraine, intelligence, social class, and familial prevalence. BMJ. 1971;2:77–81. doi: 10.1136/bmj.2.5753.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell MG, Olesen J. Increased familial risk and evidence of genetic factor in migraine. BMJ. 1995;311:541–544. doi: 10.1136/bmj.311.7004.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 26.Lipton RB, Stewart WF, Celentano DD, Reed M. Undiagnosed migraine: a comparison of symptom based and self-reported diagnosis. Arch Intern Med. 1992;152:1273–1278. doi: 10.1001/archinte.152.6.1273. [DOI] [PubMed] [Google Scholar]
- 27.Linet M, Celentano DD, Steward WF. Headache characteristics associated with physician consultation: a population-based survey. Am J Prev Med. 1991;7:40–46. [PubMed] [Google Scholar]
- 28.Ottman R, Hauser WA, Susser M. Genetic and maternal influences on susceptibility to seizures: an analytic review. Am J Epidemiol. 1985;122:923–939. doi: 10.1093/oxfordjournals.aje.a114197. [DOI] [PubMed] [Google Scholar]
- 29.Ottman R. A simple test of the multifactorial-polygenic model with sex-dependent thresholds. J Chronic Dis. 1987;40:165–170. doi: 10.1016/0021-9681(87)90068-3. [DOI] [PubMed] [Google Scholar]
- 30.Ottman R, Annegers JF, Hauser WA, Kurland LT. Higher seizure risk in offspring of mothers than of fathers with epilepsy. Am J Hum Genet. 1988;43:257–264. [PMC free article] [PubMed] [Google Scholar]
- 31.Susser M, Hauser WA, Kiely JL, Paneth N, Stein Z. Freeman JM, editor. Quantitative estimates of prenatal and perinatal risk factors for perinatal mortality, cerebral palsy, mental retardation, and epilepsy. (NIH publication no. 85-1149).Prenatal and perinatal factors associated with brain disorders. 1985:359–440. [Google Scholar]