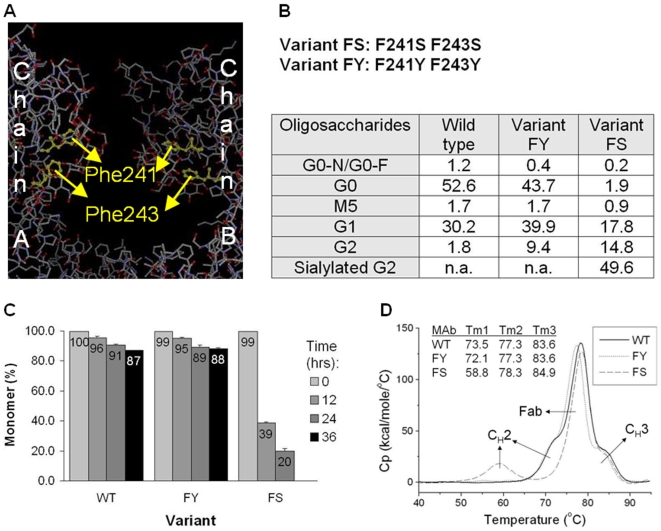
Figure 4. The IgG1 variants F241S F243S (FS) and F241Y F243Y (FY) illustrate the importance of CH-pi interactions for stability of the CH2 antibody domain.
(A) Close-up on the CH2 domain showing the location of the mutated residues on the inner side of CH2, and the alignment of Phe241 and Phe243 aromatic rings with sugar rings. (B) Glycosylation profile of wild type and the two F241 F243 variants. The percentage of the predominant glycoforms is listed. (C) Accelerated aggregation of wild type and variants at 58°C for the indicated time. Percent monomer at each time point was calculated from SEC-HPLC analysis. (D) DSC thermograms of wild type and Variants FY and FS.