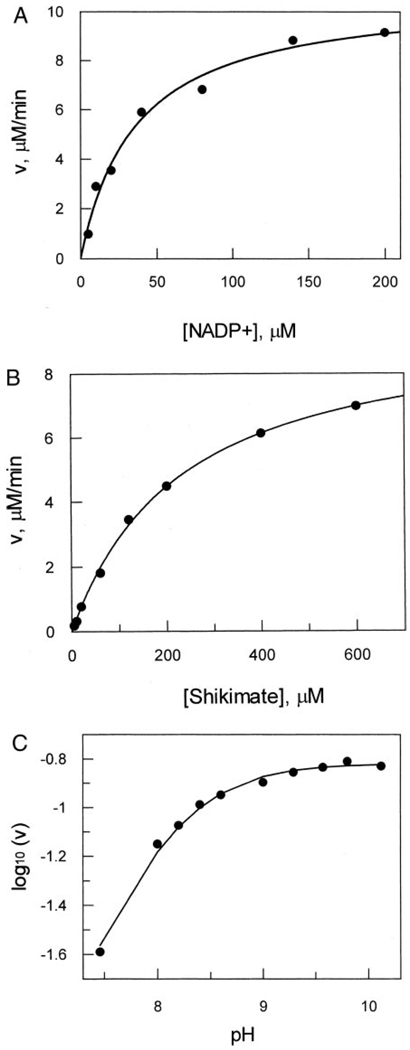
FIG. 4. Saturation kinetic profiles for the determination of wild-type HI0607 kinetic parameters.
A, increasing concentration of NADP+ with shikimate at saturation, Km(NADP+) = 34 µm. B, increasing concentration of shikimate with NADP+ at saturation, Km(shikimate) = 234 µm. The enzymatic rate, v, was calculated by monitoring the rate of reduction of NADP+ at 340 nm (ϵ = 6220 m−1 cm−1). In each case Vmax was calculated to be 10 µm/min. C, pH rate profile of HI0607 under saturating conditions for both shikimate and NADP+. The shape of the profile (with a pH optimum of 8.8 and a calculated pK of 8.1) indicates the dependence on deprotonation of a group for enzyme catalysis.