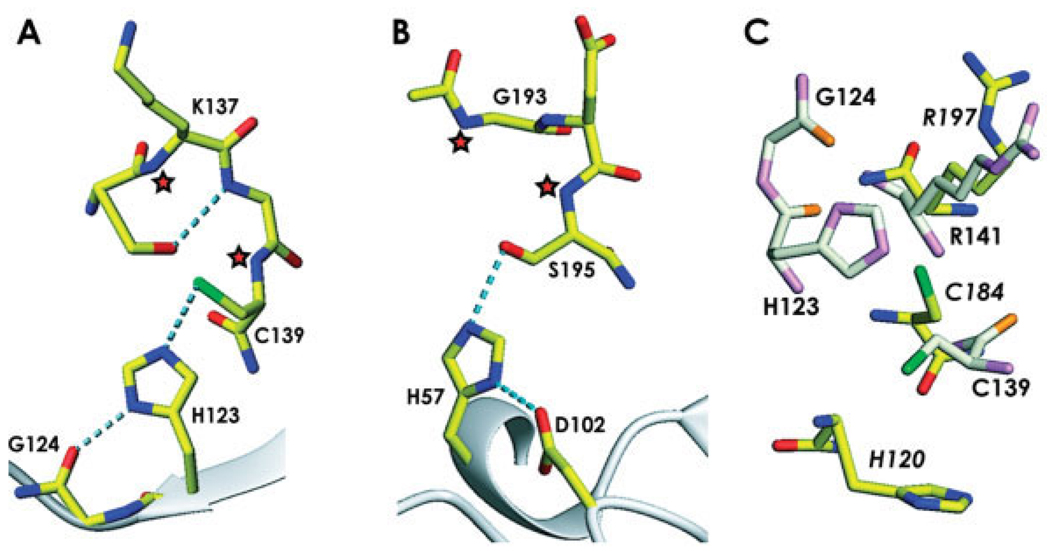
Fig. 4.
A comparison of the catalytic site of ykuD with other enzymes. (A) The ykuD catalytic triad with the side-chain of Cys139 rotated to achieve the best stereochemistry; H-bonds are shown by dotted lines, putative oxyanion hole forming backbone amides are denoted by asterisks. (B) Trypsin (PDB entry: 1YYY), with oxyanion hole indicated by asterisks. (C) Comparison of the ykuD and sortase A (PDB entry: 1T2O ) putative Cys-Arg dyads; ykuD residues are shown in light colors and the labels indicating the sortase A amino acids are in italics.