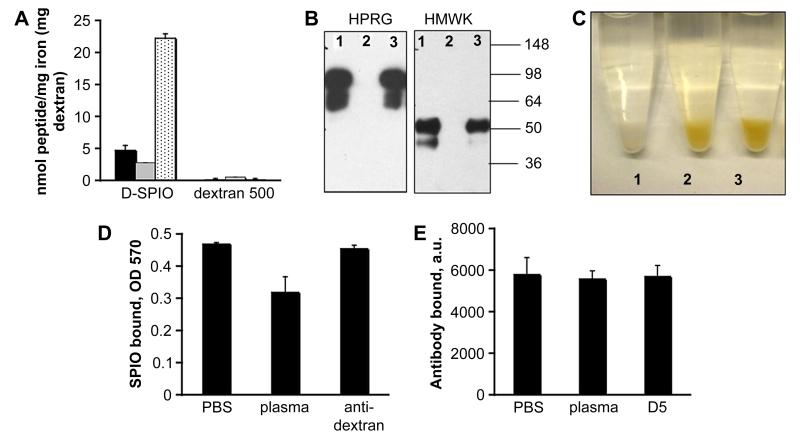
Figure 2. Probing accessibility of dextran and iron oxide on SPIO.
A, Binding of various peptide sequences to SPIO. A highly basic 34-amino acid peptide, F3 [35] (black bars), CREKA [34] (grey bars), and His-6 (dotted bars) were used. The peptides were FITC-labeled to allow quantification. SPIO were incubated with the peptides for 10 min, pelleted in an ultracentrifuge, and the amount of unbound and bound peptide was determined. As control, peptides were incubated with dextran 500 kDa (Sigma), and the amount of bound peptide was determined after separation of dextran by filter column. B, The nanoparticles were incubated with mouse plasma only (lane 1), in the presence of histidine-rich domain 5 (D5) of HMWK (lane 2) or with BSA (lane 3) for 10 min. Particle-bound proteins were eluted and separated by SDS gel electrophoresis as described in Methods. C, Binding of SPIO to D5 immobilized on glutathione-agarose. See Methods section for description of the procedure. Tube labels: 1, plain glutathione-agarose + SPIO particles (control); 2, D5-agarose + SPIO, 3, D5-agarose + SPIO +plasma. D, Binding of particles to D5-agarose was quantified by the iron assay (see Methods). SPIO were preincubated with either PBS, plasma or anti-dextran antibody before addition to the beads. Note that plasma only partially reduces the binding to D5-agarose, and that anti-dextran antibody does not interfere with the binding. Average of 3 experiments is shown. E, Binding of FITC-labeled anti-dextran antibody to SPIO. SPIO were incubated with either PBS, plasma or D5 fragment and subsequently with anti-dextran antibody. The binding of the antibody was quantified as described in Methods. The D5 treatment and plasma treatment had no effect on the antibody binding.