Abstract
The periosteum, a specialized fibrous tissue composed of fibroblast, osteoblast, and progenitor cells, may be an optimal cell source for tissue engineering based on its accessibility, the ability of periosteal cells to proliferate rapidly both in vivo and in vitro, and the observed differentiation potential of these cells. However, the functional use of periosteum-derived cells as a source for tissue engineering requires an understanding of the ability of such cells to elaborate matrix of different tissues. In this study, we subjected a population of adherent primary periosteum-derived cells to both adipogenic and osteogenic culture conditions. The commitment propensity of periosteal cells was contrasted with that of well-characterized phenotypically pure populations of NIH3T3 fibroblast and MC3T3-E1 osteoblast cell lines. Our results demonstrate that the heterogeneous populations of periosteal cells and NIH3T3 fibroblasts have the ability to express both osteoblast-like and adipocyte-like markers with similar potential. This raises the question of whether fibroblasts within the periosteum may, in fact, have the potential to behave like progenitor cells and play a role in the tissue's multilineage potential or whether there are true stem cells within the periosteum. Further, this study suggests that expanded periosteal cultures may be a source for tissue engineering applications without extensive enrichment or sorting by molecular markers. Thus, this study lays the groundwork for future investigations that will more deeply enumerate the cellular sources and molecular events governing periosteal cell differentiation.
Introduction
Periosteum-derived progenitor cells may serve as an optimal cell source for tissue engineering based on their accessibility, ability to proliferate rapidly, and capability to differentiate into multiple mesenchymal lineages.1–12 The periosteum is a specialized connective tissue that forms a fibrovascular membrane covering all bone surfaces except for that of articular cartilage, muscle, and tendon insertions, and sesamoid bones.13 Cells residing within the periosteum may be excised from any number of surgically accessible bone surfaces. In addition, when properly stimulated, the periosteum has the potential to serve as a bioreactor supporting a dramatic increase in the progenitor cell population over the course of a few days.10,13,14 Further, once cells are removed from the periosteum, they have the potential to proliferate at much higher rates than bone marrow –, cortical bone –, or trabecular bone – derived progenitor cells.15
In addition to their robust proliferation aptitude, it is well established that periosteum-derived progenitor cells have the potential to differentiate into both bone and cartilage.1,6,8,10–12,14,16–18 Further, their potential for regenerating both bone and cartilage constructs is superior to that of adipose-derived progenitor cells and comparable with that of bone marrow–derived mesenchymal stem cells.15,19 A recent study by De Bari et al. indicates that periosteal progenitor cells are able to differentiate not only into bone and cartilage cells, but also into adipocyte and skeletal myocyte cells.16 The accessibility, proliferation profile, and multi-tissue lineage capabilities of periosteal cells suggest that they may be an optimal cell source for skeletal tissue engineering; however, the ability of periosteum-derived cells to contribute to the expression and formation of multiple tissue types has yet to be elucidated from a functional tissue engineering standpoint.
The periosteum consists of two layers. The inner cambium layer is a loose collagenous matrix that houses osteoblasts, progenitor cells, and fibroblasts. It is enveloped by an outer periosteal fibrous layer containing fibroblasts as well as the blood and nerve supplies for the subperiosteal bone.20 Recent studies suggest that fibroblasts from a number of tissue sources may have the potential to commit to multiple mesenchymal lineage cell fates, including cartilage and bone.21–25 Thus, the multiple cellular types within the periosteum may have the propensity to commit to several tissue types, suggesting that the periosteum may be an advantageous cell source for functional tissue engineering with minimal ex vivo manipulation.
The purpose of this study was to establish the basic groundwork for characterizing the potential of periosteum-outgrowth cells for functional tissue engineering applications. The adherent subpopulation of primary cells grown from the periosteum was subjected to both osteogenic and adipogenic culture conditions. Using Oil Red O as a biomarker for adipogenic differentiation and alkaline phosphatase (ALP) as a maker of osteogenic differentiation, the propensity of expanded primary periosteal cells for lineage commitment was compared with known fibroblast and osteogenic cell lineages.
Materials and Methods
Periosteal cell isolation
Periosteal tissue was harvested from the tibiae of 3-week-old FVB mice by microdissection. Hind limbs were rinsed in Betadine (Fisher Scientific, Hampton, NH) and placed in PBS on ice. Using a dissection microscope, forceps, and a number 10 scalpel, the overlying skin, fascia, and muscle were carefully removed and discarded until the periosteum was exposed. The bone, with periosteum intact, was placed into a new dish with ice-cold PBS. At 3 weeks of age, the periosteum is not rigidly attached to the underlying bone, and using a scalpel and forceps, the periosteum was scored and reflected. Once reflected, the samples were moved to the sterile hood, and the periosteum was gently peeled off using forceps. Once removed, the periosteal tissue was cut into 1 mm2 pieces, and the tissue explants were cultured in 35 mm, fibronectin-coated tissue culture dishes in alpha modified minimal essential medium (αMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin and streptomycin (Invitrogen), and maintained at 37°C and 5% CO2 in a humidified incubator. After 5 to 7 days, cells had migrated out of the explanted tissue and were adhered to the tissue culture plates. At this time, the tissue explants were removed and the remaining adherent cells were cultured. Third-passage cells were used in the experiments.
Cell culture
Before experimentation, primary periosteal cells, NIH3T3 Fibroblasts (ATCC, Manassas, VA) and MC3T3-E1 osteoblasts (ATCC) were cultured in αMEM containing 10% fetal bovine serum ad 1% penicillin and streptomycin, and maintained at 37°C and 5% CO2 in a humidified incubator.
Experimental set-up
Primary mouse periosteal cells, NIH3T3 fibroblast cells, and MC3T3-E1 osteoblast-like cells were seeded at either a high density (25,000 cells/cm2) or a low density (2500 cells/cm2) in growth medium. Upon cell attachment, the medium was changed to one of three conditions: (1) osteo-inductive differentiation medium, (2) adipo-inductive differentiation medium, or (3) growth medium. In the case of osteo-inductive medium, growth medium was supplemented with 10 mM β-glycerolphosphate (Sigma, St. Louis, MO), 250 μM ascorbic-acid-2-phosphate (Sigma), and 1 μM dexamethasone (MP Biomedicals, Solon, OH). The adipo-inductive medium was supplemented with 200 μm indomethacin (MP Biomedicals), 1 μM dexamethasone (MP Biomedicals), 0.5 mM 3-isobutyl-1-methylxanthine (MP Biomedicals), and insulin 10 μg/mL (Sigma) for the first 2 days. From day 3 through day 7 growth medium was supplemented with insulin 10 μg/mL. Cells were maintained at 37°C and 5% CO2 in a humidified incubator, and medium was changed every 2 days.
Cell staining
Adipogenic differentiation and osteogenic differentiation were assessed by Oil Red O or ALP staining, respectively. Cells were fixed with citrate concentrate (Sigma) in solution with acetone. To stain lipids, cells were exposed to a working solution of Oil Red O (3 mg/mL in 99% isopropanol) (Sigma) for 6 to 15 min and cleared with 60% isopropanol. Early osteoblastic differentiation was determined by staining ALP activity. Briefly, cells were fixed for 30 s using a fixative solution of two parts Citrate working solution (2 mL Citrate concentrate [Sigma] diluted in 98 mL of deionized water) with three parts acetone. Cells were then stained by incubation with a Diazonium salt solution containing Fast Violet B salt (Sigma), distilled water, and Naphthol AS-MX phosphate alkaline solution 0.25% (Sigma). Under both staining protocols, cells were rinsed and counterstained with Mayer's Hematoxylin solution (Sigma). For controls, cells cultured in each of the three types of medium were stained for both ALP activity and lipoprotein.
Determining differentiation ratios
After staining, the cultures were examined microscopically. For each cell and medium type, micrographs were taken at random on a Nikon Eclipse TE-300 (Nikon, Melville, NY) at 20 × magnification. Using specialized software created in MATLAB (The MathWorks, Natick, MA), positively stained cells and the total number of cells in the field were counted and the fraction of differentiated cells was determined.
Statistical analysis
Differentiation fractions of primary periosteal cells, NIH3T3 fibroblasts, and MC3T3-E1 osteoblasts were compared using a difference in proportions test with confidence intervals set at 95%. p-Values less than or equal to 0.05 were considered significant. Data are presented as average differentiation percentage ± standard error.
Results
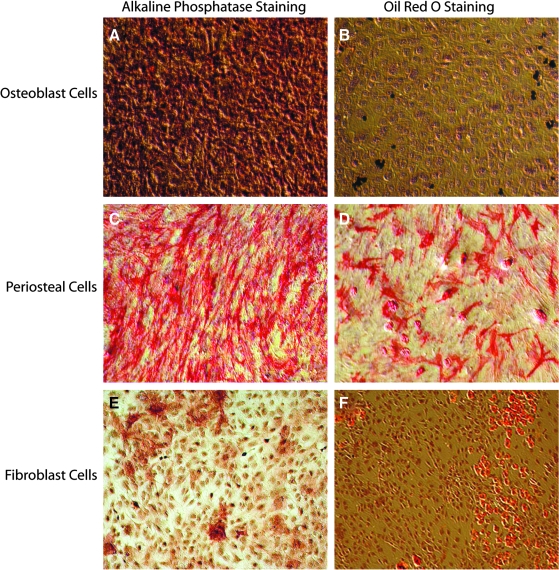
After 1 week, periosteum-derived cells and homogeneous fibroblast cultures exhibited markers indicating a propensity for both adipogenic differentiation and osteogenic differentiation, while homogeneous populations of osteoblasts expressed only osteogenic markers (Fig. 1). For all cell types, evidence of lineage commitment (both adipogenic and osteogenic) was significantly increased in cultures initially seeded at a higher density (p < 0.01).
FIG. 1.
Magnified (10×) micrographs of alkaline phosphatase (ALP)-stained cell cultures (A, C, D, E) after 1 week in osteoinductive medium and Oil red O–stained cell cultures (B, D, F) after 1 week in adipoinductive medium for all three cell types: MC3T3-E1 osteoblasts (A, B), primary periosteal cells (C, D), and NIH3T3 fibroblasts (E, F). All micrographs are of cell cultures initially seeded at high density; as in all cases, a larger percentage of positive staining was observed (p < 0.01). Panel (D) demonstrates that periosteal cells cultured in adipo-inductive medium for 1 week stain positively for ALP, suggesting that there was a subpopulation within the periosteal culture that expressed ALP in the absence of soluble osteogenic differentiation factors.
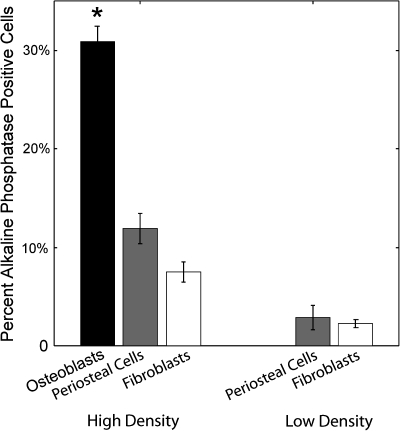
MC3T3-E1 osteoblasts had the highest level of ALP expression with 30.9% ± 1.6% of cells cultured at high density in medium promoting osteogenic differentiation staining positively (Fig. 2). However, MC3T3-E1 cells initially seeded at low density did not express ALP, even in the presence of osteo-inductive factors (Fig. 2).
FIG. 2.
Osteoblasts, periosteal cells, and fibroblasts stain positively for the osteogenic marker, ALP, when cultured at a high density in osteo-inductive medium. MC3T3-E1 osteoblasts expressed significantly (p < 0.05) higher levels of ALP activity than either periosteal cells of fibroblasts initially seeded at a high density; however, there was no expression of ALP in osteoblast cultures initially seeded at low densities. In both high- and low-density cultures, periosteal cells and NIH3T3 fibroblasts had relatively similar levels of ALP expression with no significant difference.
Similar to osteoblasts, periosteal cells expressed high levels of ALP activity in osteo-inductive cultures. Interestingly, primary periosteal cells were the only cell type to stain positively for ALP activity in adipo-inductive and growth medium cultures (Fig. 1D). The percent of cells expressing ALP in both the adipo-inductive and growth medium was not significantly different, suggesting that a basal level of cells in the primary cultures express ALP. The osteogenic potential of the progenitor cells within the periosteum was thus determined by normalizing the observed ratios of differentiation in osteo-inductive cultures with ratio of ALP expression ascertained in adipo-inductive and growth medium cultures. Thus, the percentage of periosteum-derived cells with the potential to undergo osteogenic differentiation was determined to be 11.9% ± 1.5% and 2.9% ± 1.3% for cells initially cultured at high and low density, respectively (Fig. 3).
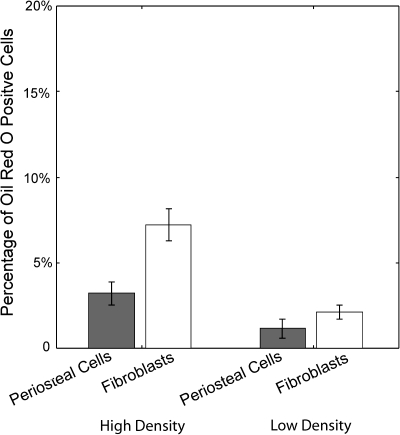
FIG. 3.
Periosteal cells and fibroblasts have a similar propensity for the adipogenic lineage when cultured for 1 week in adipo-inductive medium. MC3T3-E1 osteoblasts did not stain positively for adipogenic markers, indicating that they are a committed mature cell type. A pure population of fibroblasts has a similar potential to express adipogenic lineage markers.
Homogeneous populations of NIH3T3 fibroblasts expressed ALP activity in both high- and low-density cultures with expression levels of 7.5% ± 1.0% and 2.2% ± 0.4%, respectively (Figs. 2 and 3). The percentage of MC3T3 cells induced to express ALP was significantly higher than that of the periosteal cells and fibroblasts (p < 0.05). Interestingly, the induced ALP staining of periosteal cells was not significantly different from fibroblasts, indicating that the potential of these cells to undergo osteogenic lineage commitment was similar.
MC3T3-E1 cells did not stain positively for Oil Red O under any conditions. Primary periosteal cells underwent adipogenic differentiation when cultured with adipo-inductive soluble factors in both high- and low-density cultures resulting in 3.2% ± 0.7% and 1.2% ± 0.6% Oil Red O staining, respectively. Interestingly, the mature population of NIH3T3 fibroblasts had a relatively higher potential to undergo adipogenic lineage commitment than the periosteal cells with 7.2% ± 0.9% and a 2.1% ± 0.42% of the fibroblasts staining positively for Oil Red O in the high- and low-density cultures, respectively. The difference in induced Oil Red O staining, similar to ALP, was not statistically significant between primary periosteal cells and NIH3T3 fibroblasts, suggesting that fibroblasts and periosteal progenitor cells have similar adipogenic potential.
Discussion
In this study, we have demonstrated that the propensity for lineage differentiation of primary periosteal cells is significantly different from that of osteoblasts; however, periosteal cells exhibit a profile similar to phenotypically pure populations of fibroblasts. Under both osteo-inductive and adipo-inductive conditions, the periosteal progenitor cells and homogeneous populations of fibroblasts stained positively for cell fate markers in a density-dependent manner. These findings are consistent with the idea that upon reaching confluence, contact inhibition of cell populations results in decreased proliferation, and hence increased differentiation.26
MC3T3-E1 osteoblast cells do not have the potential to undergo adipogenic differentiation. Additionally, the ability of MC3T3-E1 cells to express osteogenic biomarkers was significantly different than that of periosteal cells or fibroblasts. Specifically, ALP staining was observed only at high initial seeding density cultures. This is consistent with the model of committed osteoblast progenitors proliferating until reaching confluence, undergoing an arrest of proliferation due to contact inhibition, and subsequently entering a program of osteogenic differentiation. Indeed, several studies have indicated that before confluence and growth arrest, MC3T3-E1 cells may fail to express ALP activity.27,28 The cells seeded at an initial low density may not have been at the stage of confluence for long enough to undergo growth arrest and increase ALP activity. In contrast, periosteal cells and fibroblasts do not appear as dependent on cell–cell contact for ALP expression.
Expanded periosteum-derived cells posses the bi-potent predisposition to both osteoblastic and adipocytic lineages in a monolayer culture, suggesting that these cells could, indeed, be a useful cell source for functional tissue engineering. Although these data are important for guiding future functional tissue engineering development exploiting the periosteum as a cell source, further molecular analysis must be conducted to fully characterize the distribution of cell types within the periosteum and their differentiation potential. Interestingly, we found that homogeneous populations of NIH3T3 fibroblasts have the ability to express both osteogenic and adipogenic biomarkers in a density-dependent manner that is remarkably similar to that of periosteal cells. This observation has been reported previously in dermal fibroblasts that possess the potential to differentiate into multiple mesenchymal lineages.21–25 However, in these studies of primary cells, it was impossible to conclude with certainty that the dermal fibroblasts themselves were multipotent or if a subpopulation of progenitor cells existed within the tissue. This study demonstrates that the homogeneous, established NIH3T3 fibroblast cell line is able to commit to multiple skeletal tissue lineages, suggesting that fibroblasts may have progenitor cell–like potential, although this remains to be confirmed at the molecular level. A recent study by Neri et al. demonstrates that NIH3T3 fibroblasts can be reprogrammed to express the embryonic stem cell–specific genes, Oct-4 and Rex-1, in the presence of embryonic stem cell extracts.29 These results, taken together, suggest that homogeneous populations of fibroblasts may be able to alter their plasticity in the presence of inductive factors.
In both osteo-inductive and adipo-inductive conditions, there was no observed difference in the potential for multi-lineage commitment of periosteal cells and fibroblasts. Specifically, it is not clear whether the multipotent capacity of periosteal tissue observed in previous studies is due to the presence of a relatively rare species of stem cells, or whether residing fibroblasts within the periosteum are able to behave like progenitor cells. Our finding that a fibroblast cell line can be induced to express markers bone and adipose biomarkers is suggestive of the latter; however, further research must be conducted to confirm this hypothesis. Specifically, ongoing work is focused on determining which molecular cell-type markers periosteum-derived cells express and which might be multi-potent through a clonal analysis. Further, although we utilized previously validated stains as osteogenic and adipogenic biomarkers, because they are more closely related to extracellular matrix and tissue function, this approach is only suggestive of cell differentiation events. The future trajectory of this research includes full characterization of the periosteal outgrowth compartment and molecular-level determination of whether cellular differentiation is, in fact, occurring. The results of this study and future studies within this central focus will have important implications for the utility of periosteal cells and fibroblasts as potential cell sources for tissue engineering therapeutics.
Acknowledgments
Research was funded by the NIH Grant AR45989, the National Science Foundation Graduate Research Fellowship, and the Department of Veteran's Affairs Pre-Doctoral Associated Health Rehabilitation Research Fellowship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Agata H. Asahina I. Yamazaki Y. Uchida M. Shinohara Y. Honda M.J. Kagami H. Ueda M. Effective bone engineering with periosteum-derived cells. J Dent Res. 2007;86:79. doi: 10.1177/154405910708600113. [DOI] [PubMed] [Google Scholar]
- 2.Emans P.J. Surtel D.A. Frings E.J. Bulstra S.K. Kuijer R. In vivo generation of cartilage from periosteum. Tissue Eng. 2005;11:369. doi: 10.1089/ten.2005.11.369. [DOI] [PubMed] [Google Scholar]
- 3.Eyckmans J. Luyten F.P. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Eng. 2006;12:2203. doi: 10.1089/ten.2006.12.2203. [DOI] [PubMed] [Google Scholar]
- 4.Hadjiargyrou M. Ahrens W. Rubin C.T. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res. 2000;15:1014. doi: 10.1359/jbmr.2000.15.6.1014. [DOI] [PubMed] [Google Scholar]
- 5.Hutmacher D.W. Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S45. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki M. Nakahara H. Nakata K. Nakase T. Kimura T. Ono K. Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor-beta and basic fibroblast growth factor. J Bone Joint Surg. 1995;77:543. doi: 10.2106/00004623-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 7.O'Driscoll S.W. Articular cartilage regeneration using periosteum. Clin Orthop . 1999;S186:203. doi: 10.1097/00003086-199910001-00020. [DOI] [PubMed] [Google Scholar]
- 8.Sakata Y. Ueno T. Kagawa T. Kanou M. Fujii T. Yamachika E. Sugahara T. Osteogenic potential of cultured human periosteum-derived cells—a pilot study of human cell transplantation into a rat calvarial defect model. J Craniomaxillofac Surg. 2006;34:461. doi: 10.1016/j.jcms.2006.07.861. [DOI] [PubMed] [Google Scholar]
- 9.Schantz J.T. Hutmacher D.W. Ng K.W. Khor H.L. Lim M.T. Teoh S.H. Evaluation of a tissue-engineered membrane-cell construct for guided bone regeneration. Int J Oral Maxillofac Implants. 2002;17:161. [PubMed] [Google Scholar]
- 10.Ueno T. Kagawa T. Fukunaga J. Mizukawa N. Sugahara T. Yamamoto T. Evaluation of osteogenic/chondrogenic cellular proliferation and differentiation in the xenogeneic periosteal graft. Ann Plast Surg. 2002;48:539. doi: 10.1097/00000637-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Wiesmann H.P. Nazer N. Klatt C. Szuwart T. Meyer U. Bone tissue engineering by primary osteoblast-like cells in a monolayer system and 3-dimensional collagen gel. J Oral Maxillofac Surg. 2003;61:1455. doi: 10.1016/j.joms.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X. Xie C. Lin A.S. Ito H. Awad H. Lieberman J.R. Rubery P.T. Schwarz E.M. O'Keefe R.J. Guldberg R.E. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20:2124. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon T.M. van Sickle D.C. Kunishima D.H. Jackson D.W. Cambium cell stimulation from surgical release of the periosteum. J Orthop Res. 2003;21:470. doi: 10.1016/S0736-0266(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 14.Ueno T. Kagawa T. Fukunaga J. Mizukawa N. Kanou M. Fujii T. Sugahara T. Yamamoto T. Regeneration of the mandibular head from grafted periosteum. Ann plast Surg. 2003;51:77. doi: 10.1097/01.SAP.0000054180.78960.15. [DOI] [PubMed] [Google Scholar]
- 15.Ng A.M. Saim A.B. Tan K.K. Tan G.H. Mokhtar S.A. Rose I.M. Othman F. Idrus R.B. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Orthop Sci. 2005;10:192. doi: 10.1007/s00776-004-0884-2. [DOI] [PubMed] [Google Scholar]
- 16.De Bari C. Dell'Accio F. Vanlauwe J. Eyckmans J. Khan I.M. Archer C.W. Jones E.A. McGonagle D. Mitsiadis T.A. Pitzalis C. Luyten F.P. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara H. Bruder S.P. Goldberg V.M. Caplan A.I. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop. 1990;223:32. [PubMed] [Google Scholar]
- 18.Nakahara H. Bruder S.P. Haynesworth S.E. Holecek J.J. Baber M.A. Goldberg V.M. Caplan A.I. Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone. 1990;11:181. doi: 10.1016/8756-3282(90)90212-h. [DOI] [PubMed] [Google Scholar]
- 19.Park J. Gelse K. Frank S. von der Mark K. Aigner T. Schneider H. Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med. 2006;8:112. doi: 10.1002/jgm.826. [DOI] [PubMed] [Google Scholar]
- 20.Ellender G. Feik S.A. Carach B.J. Periosteal structure and development in a rat caudal vertebra. J Anat. 1988;158:173. [PMC free article] [PubMed] [Google Scholar]
- 21.Cui L. Yin S. Deng C.L. Yang G.H. Chen F.G. Liu W. Liu D.L. Cao Y.L. Cartilage-derived morphogenetic protein 1 initiates chondrogenic differentiation of human dermal fibroblasts in vitro. Zhonghua Yi Xue Za Zhi. 2004;84:1304. [PubMed] [Google Scholar]
- 22.Mizuno S. Glowacki J. Three-dimensional composite of demineralized bone powder and collagen for in vitro analysis of chondroinduction of human dermal fibroblasts. Biomaterials. 1996;17:1819. doi: 10.1016/0142-9612(96)00041-5. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno S. Glowacki J. Chondroinduction of human dermal fibroblasts by demineralized bone in three-dimensional culture. Exp Cell Res. 1996;227:89. doi: 10.1006/excr.1996.0253. [DOI] [PubMed] [Google Scholar]
- 24.Rutherford R.B. Moalli M. Franceschi R.T. Wang D. Gu K. Krebsbach P.H. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- 25.Sorisky A. Pardasani D. Gagnon A. Smith T.J. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J clin Endocrinol Metab. 1996;81:3428. doi: 10.1210/jcem.81.9.8784110. [DOI] [PubMed] [Google Scholar]
- 26.Shigematsu M. Watanabe H. Sugihara H. Proliferation and differentiation of unilocular fat cells in the bone marrow. Cell Struct Funct. 1999;24:89. doi: 10.1247/csf.24.89. [DOI] [PubMed] [Google Scholar]
- 27.Quarles L.D. Yohay D.A. Lever L.W. Caton R. Wenstrup R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7:683. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- 28.Wang D. Christensen K. Chawla K. Xiao G. Krebsbach P.H. Franceschi R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 29.Neri T. Monti M. Rebuzzini P. Merico V. Garagna S. Redi C.A. Zuccotti M. Mouse fibroblasts are reprogrammed to Oct-4 and Rex-1 gene expression and Alkaline Phosphatase activity by embryonic stem cell extracts. Cloning Stem Cells. 2007;9:394. doi: 10.1089/clo.2006.0011. [DOI] [PubMed] [Google Scholar]