Table 2.
Dienamide formation from enyne 1 and isocyanates 2a-2j
| entry | E/Z products | E:Z ratioa | rxn temp | % yieldb |
|---|---|---|---|---|
| 1 | 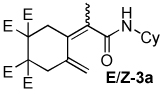 |
5:1 | rt, 1 h | 70 |
| 2 | 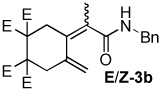 |
2:1 | rt, 2 h | 68 |
| 3 | 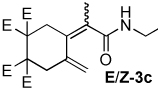 |
2:1 | 80 °C, 1 h | 75 |
| 4 | 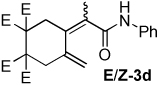 |
2:1 | 60 °C, 2 h | 79 |
| 5 | 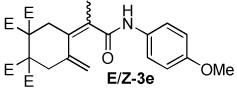 |
2:1 | 60 °C, 2 h | 74 |
| 6 | 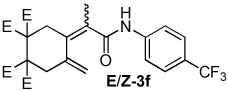 |
1.8:1 | 100 °C, 2 h | 57 |
| 7 | 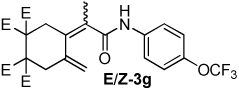 |
2:1 | 80 °C, 5 h | 66 |
| 8 | 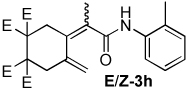 |
2:1 | 80 °C, 1 h | 71 |
| 9 | 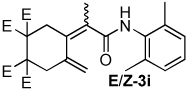 |
2:1 | 80 °C, 1.5 h | 80 |
Reaction conditions: 1 equiv 1a, 1 equiv 2,
Determined by 1H-NMR,
isolated yields, average of 2 runs
