Abstract
Background
Bariatric surgery remains the most effective modality to induce sustainable weight loss in the morbidly obese. Our aim was to compare outcomes between the laparoscopic Roux-en-Y gastric bypass (LRYGBP) and the laparoscopic adjustable gastric banding device (LAGBD) method with 5-year follow-up in a Canadian bariatric surgery centre.
Methods
This is a retrospective outcomes analysis of 1035 laparoscopic bariatric procedures performed over 7 years. We extracted data from our prospectively collected bariatric surgery registry from Feb. 1, 2002, to Jun. 30, 2008. We evaluated patient demographics, weight loss, complications, mortality and need for revision surgery by procedure type.
Results
We examined outcomes in 149 (14.4%) LAGBD and 886 (85.6%) LRYGBP procedures. The mean body mass index (BMI) was significantly higher in the LRYGBP group (50.9, standard deviation [SD] 8.9, v. 45.0, SD 6.7) whereas age and sex ratio were the same. There were 3 deaths (0.3%) in the LRYGBP group and no deaths in the LAGBD group. Sixteen patients (10.8%) in the LAGBD group needed conversion to LRYGBP because of poor weight loss, band intolerance, band erosion or slippage, and 6 patients (0.7%) in the LRYGBP group required revision because of inability to achieve the desired weight loss. The percent excess-weight loss was 41, 49, 59, 60 and 61 at 1, 2, 3, 4 and 5 years postsurgery for the LAGBD patients who kept their band, and 70, 79, 79, 79 and 75 for the LRYGBP patients.
Conclusion
Laparoscopic weight loss surgery can be performed safely with acceptable mortality. Our study suggests superior weight loss and low revision requirement for the LRYGBP, making this a more durable procedure in a publicly funded health care system.
Abstract
Contexte
La chirurgie bariatrique demeure le moyen le plus efficace de provoquer une perte de poids durable chez les patients atteints d’obésité morbide. Nous voulions comparer les résultats du pontage gastrique Roux-en-Y par laparoscopie (PGRYL) à ceux de la méthode de l’anneau gastrique ajustable par laparoscopie (AGAL) avec un suivi sur 5 ans dans un centre canadien de chirurgie bariatrique.
Méthodes
Il s’agit d’une analyse rétrospective des résultats de 1035 interventions bariatriques par laparoscopie pratiquées en 7 ans. Nous avons extrait des données de notre registre prospectif de chirurgie bariatrique pour la période du 1 février 2002 au 30 juin 2008. Nous avons évalué les caractéristiques démographiques des patients, la perte de poids, les complications, la mortalité et le besoin d’une chirurgie de révision selon le type d’intervention.
Résultats
Nous avons analysé les résultats de 149 (14,4 %) interventions AGAL et 886 (85,6 %) interventions PGRYL. L’indice de masse corporelle (IMC) était beau-coup plus élevé chez les patients du groupe PGRYL (50,9, écart-type [ET] 8,9, c. 45,0, ET 6,7) tandis que les ratios d’âge et de sexe étaient les mêmes. Il y a eu 3 décès (0,3 %) chez les patients du groupe PGRYL et aucun chez ceux du groupe AGAL. Dans le groupe AGAL, 16 (10,8 %) des patients ont dû recevoir une conversion à la méthode PGRYL à cause de la faible perte de poids, de l’intolérance de l’anneau, de l’érosion ou du glissement de l’anneau, et dansle groupe PGRYL, 6 (0,7 %) patients ont eu besoin d’une révision parce qu’ils n’ont pu perdre autant de poids qu’ils le souhaitaient. Après 1, 2, 3, 4 et 5 ans suivant l’intervention chirurgicale, le pourcentage de perte de poids excédentaire s’est établi à 41, 49, 59, 60 et 61 chez ceux qui ont subi l’intervention AGAL et à 70, 79, 79, 79 et 75 chez les patients qui ont subi une intervention de type PGRYL.
Conclusion
L’intervention chirurgicale par laparoscopie pour perte de poids peut être pratiquée en toute sécurité avec un taux de mortalité acceptable. Notre étude indique que la méthode PGRYL fait perdre plus de poids et exige peu de révisions, ce qui en fait une intervention plus viable dans un système de santé public.
Obesity is now recognized as a chronic disease with multiple associated disorders.1 According to the World Health Organization, obesity is reaching epidemic proportions with more than 1 billion adults who are overweight, 300 million who have class I or II obesity and 30 million who have class III obesity, defined as a body mass index (BMI) greater than 40 kg/m2 (also referred to as morbid obesity).2,3 Canada is no exception to this epidemic, as most of the Canadian population is overweight or obese4 and 2% of men and 4% of women (~900 000 total) are morbidly obese.5 Rates of obesity-related deaths are at least on par with rates of smoking-related deaths, and some authors believe that obesity is now the number one killer in North America.6
The nonsurgical treatment of severe obesity is a lifelong struggle with high recidivism and suffering.7 Whereas no one disputes the ability of morbidly obese patients to lose weight,8 the challenge is to maintain weight loss in the long term.9–11 Such weight-loss maintenance is critical to achieving the beneficial effects of a reduced weight. Bariatric surgery is the only treatment modality that produces significant, sustained, long-term weight loss in patients with severe obesity.12,13 In addition, permanent weight loss through bariatric surgery reduces the relative risk of death by 35% to 89%, depending on the study,14–18 and produces significant pharmacoeconomic benefits.19
Since the first laparoscopic Roux-en-Y gastric bypass20 (LRYGBP), the technique has rapidly evolved and currently represents the preferred surgical procedure for weight loss in North America. Since the Food and Drug Adinistration approval of the laparoscopic adjustable gastric banding device (LAGBD) in 2001 in the United States, the LAGBD method has been gaining popularity as an alternative bariatric procedure.21 The McGill University bariatric surgery program has performed all bariatric procedures with open laparotomy since 1963. Our minimally invasive bariatric surgery experience began on the Feb. 8, 2002, when we successfully completed our first laparoscopic gastric bypass. We added the LAGBD method shortly thereafter. To date, our unit has performed more than 1000 laparoscopic bariatric procedures. In this study, we aimed to assess our 5-year outcomes with LRYGBP and the LAGBD method.
Methods
This was a retrospective analysis of a prospectively maintained bariatric surgery registry at the McGill University Health Centre of all patients who underwent LRYGBP and LAGBD surgery from February 2002 to June 2008. All patients met the requirements of the 1991 National Institute of Health Consensus Conference guidelines22 for bariatric surgery, specifically, a BMI of 35–39 kg/m2 with associated comorbidities, or BMI 40 kg/m2 or greater. A multidisciplinary team performed medical, nutritional and psychological assessments of all patients. Uncontrollable binge eating disorders required treatment before surgery. All patients were required to manifest an understanding of the surgical procedure they were scheduled to undergo, its mechanism of weight loss, and potential long- and short-term complications, as well as an understanding of the requirements for dietary and physical activity for each procedure, lifelong nutritional and vitamin supplements, and follow-up. The choice of procedure was left to the patient after initial (and subsequent, if needed) consultation with the bariatric surgeon, which included a detailed formal presentation of the anatomy, mechanisms of action, short- and long-term complication rates, and expected weight loss from each procedure.
We used a detailed patient questionnaire to obtain patient demographics and information about patients’ past attempts at weight loss, obesity-associated conditions, previous surgery and current medications, and we verified this information at the initial consultation. Initial body weight and height were measured in the office, and a BMI was calculated. Data on subsequent weight loss were obtained by direct measurement at our bariatric clinic or from the reports submitted by the patient’s physician. All subsequent complications and reoperations were recorded in our electronic registry. The percent excess-weight loss (%EWL) was calculated as 100% × ([W0–Wi]/EW0), where W0 is the weight (kg) at the time of surgery, Wi is the weight (kg) at the last follow-up, and EW0 is the excess weight at the time of surgery. We estimated excess weight according to the formula described by Deitel and Green-stein23 and defined excess weight based on the Metropolitan tables for middle frame individuals.24 We defined complications occurring within 30 days from the date of surgery as short-term complications, and those occurring after 30 days as long-term complications. We also determined the 90-day and long-term mortalities that could be related to the original bariatric surgery.
Our LRYGBP technique has been described elsewhere25 and involves a 30–50 cm biliopancreatic limb and a 100-cm retrocolic, antegastric, alimentary limb. The surgeon constructs the jejunojejunal anastomosis side-to-side with a single firing of a linear endostapler, and hand sews the defect. The gastric pouch is small (1.5 × 5.0 cm) and vertically oriented, and the gastrojejunal anastomosis is hand sewn. The Petersen space and the transverse mesocolic defect are routinely closed with polypropelene sutures. We insert all the LAGBDs via the pars flaccida technique, and perform band adjustments in the office by direct puncture of the port. The first adjustment is performed 6 weeks after surgery and the subsequent adjustments according to the patient’s weight loss, satiety and gastrointestinal symptoms. Patients with insufficient weight loss are referred for dietician review. Upper gastrointestinal contrast studies are requested if a problem with the band is suspected clinically.
We provided all patients in our study with an operation-specific information kit outlining detailed postoperative diet plans and activity regimens. We encouraged all patients to wear an accelerometer (pedometer) and to maintain an activity level of at least 10 000 steps per day or the equivalent. They were given follow-up appointments at 14, 30, 90 and 180 days, and at 6–12 months thereafter. All attempts were made to achieve 100% follow-up.
We used SPSS 14.0 for the computations and statistical analysis. We tested continuous variables for significance using unpaired t tests, and used χ2 or Fisher exact tests to compare proportions as appropriate.
Results
In total, 1035 patients underwent laparoscopic bariatric surgery, and, of those, 886 (85.6%) underwent LRYGBP and 149 (14.4%) the LAGBD procedure. For the LAGBD group, we performed 115 of the procedures using Swedish Quick Close bands (Ethicon Endo-Surgery Canada) and 34 using the Lap-Band System (Allergan Canada). The mean age for all patients was 40.4 (range 14–74) years and the mean BMI 50.2 (range 33–107) kg/m2. The demographics of the patients in both groups are shown in Table 1. Patients in the LRYGBP group were slightly younger by 2 years with significantly higher BMIs and more excess weight compared with the LAGBD patients. The operating time (skin-to-skin) was shorter for the LAGBD group. There was 1 unplanned conversion to laparotomy in the LRYGBP group. In the first 50 cases, we observed 80% or greater (depending on bed availability) of the LRYGBP cohort in an intensive care unit step-down bed. As we became more skilled at the surgery and the intensive care unit resources became scarce, we admitted these patients to the general wards. Length of stay is thus calculated after the first 100 LRYGBP patients. It is our policy to observe all our LAGBD patients in a hospital setting overnight, which accounts for the about 19 (standard deviation 5) hours length of stay in the LAGBD cohort compared with the 42 (standard deviation 8) hours length of stay in the LRYGBP cohort.
Table 1.
Demographic details and starting weights and body mass indices of patients who underwent the laparoscopic Roux-en-Y gastric bypass and the laparoscopic adjustable gastric banding device procedure
| Characteristic | Patient group; mean (SD) [range]* | p value | |
|---|---|---|---|
| LRYGBP, n = 886 | LAGBD, n = 149 | ||
| Women:men, no. (%) | 641:235 (72.3:27.7) | 106:43 (72.3:27.7) | |
| Age, yr | 40.1 (10.1) [17–70] | 42.3 (11.6) [14–74] | 0.007 |
| Weight, kg | 145.1 (30.9) [93.2–290.9] | 126.3 (22.8) [78.6–225.1] | < 0.001 |
| BMI, kg/m2 | 50.9 (8.9) [36–107] | 45.0 (6.7) [33–74] | < 0.001 |
| Excess weight, kg | 76.9 (27.7) [28.2–217.7] | 60.9 (22.7) [17.3–137.7] | < 0.001 |
| OR time, skin-to-skin, min | 78.2 (7.8) | 52.1 (4.7) | < 0.001 |
| Length of stay, h† | 42 (8) | 19 (5) | < 0.001 |
BMI = body mass index; LAGBD = laparoscopic adjustable gastric banding device; LRYGBP = laparoscopic Roux-en-Y gastric bypass; OR = operating room; SD = standard deviation.
Unless otherwise indicated.
This was calculated after the first 100 LRYGBP patients. It is our policy to observe LAGBD patients overnight in a hospital setting.
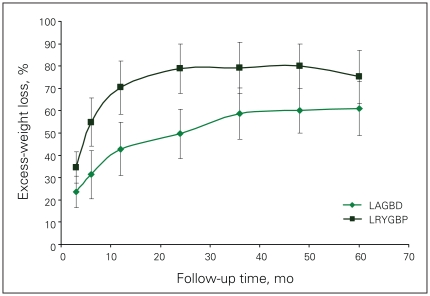
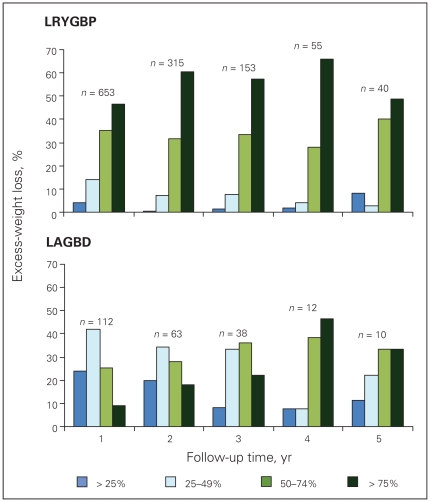
Table 2 shows the follow-up rate at each time point, and the mean weight loss in kg, the mean BMI and the %EWL of the 2 groups. We have made every effort possible, given the limited bariatric clinic staff, to follow-up all our patients for life. This included follow-up phone calls and emails by our single bariatric nurse clinician and receptionist, communication with patients’ physicians, or, in desperate circumstances, asking the local police department for help in tracing the patients. Despite these efforts, we were unable to follow up one-third of our patients after 2–3 years. The main reasons were lack of patient adherence, patient relocation and loss of contact information. There was no statistical difference in the follow-up rates of the 2 cohorts. Patients in the LRYGBP group had more weight loss, lower BMI and %EWL at each time point. The nadir of weight loss occurred at 2 years with the LRYGBP group, with weight regain and stabilization subsequently. The LAGBD group showed continual weight loss up to 5 years, where partial data are available because some patients were lost to follow-up. The rate of loss to follow-up is similar between band and bypass patients. Figure 1 shows the %EWL of patients followed up at each time point up to 5 years. Patients in the LAGBD group who had their band removed were not included in the analysis from the time of band removal onward. The LRYGBP group shows significantly increased %EWL at each time point, averaging 15% greater than the LAGBD group. Figure 2 shows the %EWL within 25% cut-off points. The LRYGBP group demonstrated a significantly higher proportion of patients in the upper quartiles of excess-weight loss.
Table 2.
The mean weight loss, mean body mass index, percentage of excess-weight loss and the patient follow-up rate at each time point
| Procedure; variable | Follow-up; mean (SD)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | 6 yr | 7 yr | |
| Gastric bypass | |||||||||
| Weight loss, kg | 12.2 (6.7) | 25.7 (11.2) | 52.6 (19.6) | 60.2 (18.3) | 59.3 (25.2) | 56.3 (20.2) | 52.6 (19.8) | 49.6 (18.2) | 48.7 (16.3) |
| BMI, kg/m2 | 42.2 (8.5) | 36.8 (8.8) | 32.8 (7.8) | 30.4 (7.6) | 30.4 (6.8) | 30.3 (7.3) | 30.5 (8.5) | 29.6 (6.1) | 29.9 (8.0) |
| Excess-weight loss, % | 34.6 (14.6) | 54.8 (20.4) | 70.4 (22.5) | 78.8 (19.3) | 79.2 (22.1) | 78.9 (21.9) | 75.2 (24.5) | 78.8 (21.9) | 76.2 (28.4) |
| No. of patients examined | 886 | 712 | 653 | 315 | 153 | 55 | 40 | 35 | 10 |
| % of eligible patients followed up | 100 | 97 | 83 | 62 | 62 | 71 | 67 | 50 | 55 |
| Gastric band | |||||||||
| Weight loss, kg | 8.7 (5.3) | 13.9 (7.1) | 18.5 (10.5) | 24.5 (13.5) | 30.1 (12.2) | 34.6 (15.6) | 35.2 (10.5) | 18.4 (6.7) | — |
| BMI, kg/m2 | 40.4 (5.6) | 38.7 (5.4) | 36.2 (6.1) | 34.8 (6.3) | 32.7 (6.1) | 30.4 (7.1) | 31.1 (4.4) | 39.2 (2.1) | — |
| Excess-weight loss, % | 23.6 (14.6) | 31.4 (17.2) | 42.8 (23.4) | 49.6 (24.6) | 58.6 (24.0) | 60.0 (20.3) | 61 (23.1) | 26.6 (4.7) | — |
| No. patients examined | 149 | 132 | 112 | 63 | 38 | 12 | 10 | 2 | — |
| % of eligible patients followed up | 100 | 98 | 73 | 66 | 72 | 66 | 80 | 50 | — |
BMI = body mass index; SD = standard deviation.
Unless otherwise indicated.
Fig. 1.
The percentage of excess-weight loss of patients in the laparoscopic Roux-en-Y gastric bypass (LRYGBP) and laparoscopic adjustable gastric banding device (LAGBD) groups (after 2 years, we were unable to follow-up one-third of patients).
Fig. 2.
The proportion of patients achieving the targeted percentage of excess-weight loss at each time point. The actual number of patients with available data at each time point is indicated (after 2 years, we were unable to follow-up one-third of patients). LRYGBP = laparoscopic Roux-en-Y gastric bypass; LAGBD = laparoscopic adjustable gastric banding device.
Table 3 lists the complications observed in this study. These are separated into short-term complications (within 30 d postsurgery) or long-term complications (occurring after 31 d postsurgery). The complications that led to re-operation are listed as well. Complications occurred in 35 (23.5%) of LAGBD cases and in 135 (15.2%) of the LRYGBP cohort (significantly fewer than in the LAGBD cohort, χ2 = 4.17, p = 0.041). In the LAGBD cohort, 11 (7.3%) short-term complications were observed, with none requiring reoperation. The 74 (8.4%) short-term complications in the LYRGBP cohort (χ2 = 0.06, p = 0.86) required 22 reoperations and 10 percutaneous drainage interventions to treat them. There were 24 (16.1%) long-term complications in the LAGBD cohort. All but 1 required reoperation. These reoperations were all carried out laparoscopically, except for the port revisions that required change of the port under short-duration general anesthesia. In one band leak, the patient elected not to have this corrected and has retained his leaking band. There were 6 band erosions (4.0%), 3 band leaks (2.0%) and 4 band slippages (2.7%). The 7 patients who did not tolerate their bands and/or did not lose weight were included as patients with long-term complications because reoperation was performed to deal with the problem as identified by the patient. The 61 (6.8%) cases involving long-term complications in the LRYGBP cohort (significantly fewer than in the LAGBD cohort, χ2 = 9.8, p = 0.002) required 27 (3.0%) reoperations to treat them. This included 3 perforations after endoscopic dilatation of strictures of the gastrojejunostomy. The remaining 30 endoscopic/radiologic dilatations of gastrojejunostomy strictures were successful without perforation. All but 1 reoperations in the LRYGBP cohort were carried out laparoscopically, with 1 conversion to laparotomy in the patient with jejunojejunal intrasusception.
Table 3.
Types and frequencies of complications for each type of surgery, and types and frequencies of complications that precipitated additional surgeries
| Complication | LAGBD patients, n = 149 |
LRYGBP patients, n = 886 |
||
|---|---|---|---|---|
| No. (%) | No. (%) requiring reoperation/intervention | No. (%) | No. (%) requiring reoperation/intervention | |
| Short-term complication | ||||
| Abdominal abscess | 0 | 0 | 3 (0.3) | 2 (1 percutaneous drainage) |
| Abdominal pain NYD | 2 (1.3) | 0 | 2 (0.2) | 0 |
| Acute renal failure | 0 | 0 | 1 (0.1) | 0 |
| Acute small bowel obstruction | 0 | 0 | 1 (0.1) | 0 |
| Anastomotic bleed | NA | NA | 5 (0.6) | 0 |
| Anastomotic leak | NA | NA | 27 (3.0) | 15 (9 percutaneous drainage, 3 conservative) |
| Antiperistaltic roux limb | NA | NA | 1 (0.1) | 1 |
| Band port site infection | 2 (1.3) | 0 | NA | NA |
| Cirrhosis at surgery | 0 | 0 | 2 (0.2) | 0 |
| Conversion to laparotomy | 0 | 0 | 1 (0.1) | 0 |
| Deep vein thrombosis | 0 | 0 | 1 (0.1) | 0 |
| Fever NYD | 1 (0.7) | 0 | 5 (0.6) | 4 |
| Liver laceration | 2 (1.3) | 0 | 4 (0.5) | 0 |
| Mortality within 30 days of surgery | 0 | NA | 3 (0.3) | NA |
| Neurapraxia arm | 0 | 0 | 1 (0.1) | 0 |
| Pancolitis | 0 | 0 | 1 (0.1) | 0 |
| Pancreatitis | 0 | 0 | 1 (0.1) | 0 |
| Pulmonary edema | 0 | 0 | 1 (0.1) | 0 |
| Pulmonary embolism | 0 | 0 | 1 (0.1) | 0 |
| Small bowel perforation | 0 | 0 | 1 (0.1) | 0 |
| Splenic laceration | 1 (0.76) | 0 | 1 (0.1) | 0 |
| Stomal ulcer | NA | 0 | 6 (0.7) | 0 |
| Technical problems intraoperatively | 1 (0.76) | 0 | 4 (0.5) | 0 |
| Trochar site infection | 2 (1.3) | 0 | 1 (0.1) | 0 |
| Total | 11 (7.3) | 0 | 74 (8.4) | 32 (3.6) |
|
Long-term complication | ||||
| Adjustment port revisions | 4 (2.7) | 4 | NA | 0 |
| Anastomotic leak/gastrogastric fistula | NA | NA | 4 (0.5) | 4 |
| Band erosion | 6 (4.0) | 6 | NA | 0 |
| Band intolerance/inability to lose weight | 7 (4.7) | 7 | NA | 0 |
| Band leak | 3 (2.0) | 2 | NA | 0 |
| Band slipage | 4 (2.7) | 4 | NA | 0 |
| Bowel obstruction | 0 | 0 | 2 (0.2) | 2 |
| Cancer diagnosed at follow-up | 0 | 0 | 5 (0.6) | 5 |
| Cholelithiasis | 0 | 0 | 6 (0.7) | 4 |
| Hypoglycemic episodes | 0 | 0 | 1 (0.1) | 0 |
| Internal hernia | 0 | 0 | 6 (0.7) | 6 |
| Jejuno–jenunostomy intrasusception | NA | NA | 1 (0.1) | 1 |
| Partial obstruction jejunojenunostomy | NA | NA | 3 (0.3) | 2 |
| Stenosis of the gastrojejunostomy | NA | NA | 33 (3.7) | 3 |
| Total | 24 (16.1) | 23 (15.4) | 61 (6.8) | 27 (3.0) |
LAGBD = laparoscopic adjustable gastric banding device; LRYGBP = laparoscopic Roux-en-Y gastric bypass; NA = not applicable; NYD = not yet diagnosed.
There were no deaths in the LAGBD group. There were 3 deaths in the LRYGBP group, and these are listed in Table 4, along with the complications presumed to be the causes of death. The sequence number indicates the position of the particular patient in the order of performance of his or her operation, with 1 being the first case. The first female patient who died (sequence no. 45) developed a large liver laceration from aggressive manipulation of the liver retractor, which was controlled by packing. Deep vein thrombosis prophylaxis was withheld in the postoperative period, and the patient collapsed and died from a massive pulmonary embolus on the way out of the hospital on her day of discharge, 4 days after surgery. The male patient (sequence no. 507) with anastomotic leak developed shortness of breath on the first postoperative day; a myocardial infarction was suspected and he was transferred to the intensive care unit and treated for an infarct. He died 2 days later. At autopsy, a contained anastomotic leak was found, which we attributed as contributing to his death. Though the cause of death was listed as a myocardial infarction, we believe this patient succumbed from multiple organ failure secondary to his leak. The young female patient (sequence no. 662) who developed an anastomotic leak received prompt laparoscopic repair and drainage (within 36 h) but succumbed to unrelenting progressive organ failure within 76 hours of the original surgery despite aggressive critical care support (including activated protein C). This patient’s case is detailed elsewhere.26 Fisher exact test analysis of the deaths within each surgery group shows p = 1.0, indicating that the type of surgery did not significantly influence mortality risk.
Table 4.
Details of the 3 deaths following laparoscopic Roux-en-Y gastric bypass
| Sex | Age, yr | BMI | Sequence number | Complication | Cause of death |
|---|---|---|---|---|---|
| F | 56 | 56.8 | 45 | Massive liver laceration | Pulmonary embolism |
| M | 55 | 51.0 | 507 | Anastomotic leak | Myocardial infarction |
| F | 30 | 56.7 | 662 | Anastomotic leak | Multiple organ failure |
BMI = body mass index; F = female; M = male.
Table 5 shows the proportion of patients who still had a BMI greater than 35 kg/m2 at 3 years postsurgery and those with %EWL less that 50%. If we accept the definition of successful result after bariatric surgery as weight loss greater than 50% of the excess weight, LRYGBP demonstrates a superior outcome in comparison with the LAGBD procedure. To adjust for the lack of 100% follow-up at 3 years, we assigned all patients lost to follow-up as not having lost any weight. The analysis showed p = 0.005 favouring the LRYGBP cohort. On the other hand, the same “failure rate” based on BMI being at a level of morbid obesity 3 years after surgery (> 35 kg/m2) shows no statistical difference between the 2 groups, even after adjusting for patients lost to follow-up. There were 6 patients in the LRYGBP group who were unsatisfied with their BMI after 3 or more years postsurgery who requested revisional surgery. Table 6 lists their characteristics, and all underwent a revision of their standard LRYGBP to a distal revision of the jejuno–jejunostomy to create a common channel of 100 cm. The results of this distal bypass to date are not very favourable. There were 16 band explants in the LAGBD group for the indications listed in Table 7. Unlike the distal gastric bypass revisions, the conversion of LAGBD patients who did not lose weight to LRYGBP surgery resulted in more favourable outcomes given the short follow-up periods.
Table 5.
Proportion of patients who had not reached a body mass index of less than 35 kg/m2 or a percentage of excess-weight loss greater than 50% at the 3-year follow-up*
| Variable at 3 years | Group; no. (%) of patients | Odds ratio (95% CI) | p value | |
|---|---|---|---|---|
| LRYGBP | LAGBD | |||
| BMI > 35 kg/m2 | 32/143 (22) | 12/36 (33) | 0.6 (0.2–1.3) | 0.25 |
| EWL < 50% | 13/143 (9) | 14/36 (38) | 6.4 (2.4–16.8) | < 0.001 |
BMI = body mass index; CI = confidence interval; EWL = excess-weight loss; LAGBD = laparoscopic adjustable gastric banding device; LRYGBP = laparoscopic Roux-en-Y gastric bypass.
We were unable to follow up one-third of patients in each group at the 3-year follow-up or later.
Table 6.
Characteristics of patients who underwent revision surgery after laparoscopic gastric bypass for inadequate weight loss
| Patient ID | Sex | Start BMI | BMI at LRYGBP revision | Reason for LRYGBP revision | Years since LRYGBP surgery | Revisional procedure | BMI at last follow-up visit (time in years) |
|---|---|---|---|---|---|---|---|
| 1 | F | 70 | 64 | Did not lose target weight | 3 | Distal LRYGBP | 56 (2.0) |
| 2 | F | 44 | 43 | Did not lose target weight | 6 | Distal LRYGBP | 43 (0.1) |
| 3 | F | 77 | 54 | Did not lose target weight | 3 | Distal LRYGBP | 52 (0.3) |
| 4 | F | 65 | 49 | Did not lose target weight | 3 | Distal LRYGBP | 47 (1.0) |
| 5 | M | 55 | 54 | Did not lose target weight | 4 | Distal LRYGBP | 50 (2.0) |
| 6 | F | 61 | 42 | Did not lose target weight | 3.5 | Distal LRYGBP | 40 (0.3) |
BMI = body mass index; F = female; M = male; LRYGBP = laparoscopic Roux-en-Y gastic bypass.
Table 7.
Characteristics of the patients who underwent band explantation and type of revision surgery
| Patient no. | Sex | Start BMI | BMI at band explantation | Reason for band explantation | Years since band surgery | Revisional procedure | BMI at last follow-up visit (time in years) |
|---|---|---|---|---|---|---|---|
| 1 | F | 41 | 30 | Band intolerance | 5 | LRYGBP | 27 (0.3) |
| 2 | M | 40 | 40 | Band erosion | 0.1 | None | 45 (0.1) |
| 3 | M | 43 | 41 | Band leakage | 0.5 | None | NA |
| 4 | M | 48 | 42 | Band erosion | 0.5 | LRYGBP | 34 (2.0) |
| 5 | F | 46 | 42 | Band erosion | 0.3 | None | NA |
| 6 | M | 46 | 43 | Did not lose target weight | 4 | LRYGBP | 26 (2.0) |
| 7 | F | 47 | 39 | Did not lose target weight | 1.5 | LRYGBP | 33 (0.5) |
| 8 | M | 48 | 49 | Did not lose target weight | 1 | LRYGBP | 35 (2.0) |
| 9 | F | 38 | 38 | Did not lose target weight | 2 | LRYGBP | 27 (0.5) |
| 10 | F | 46 | 43 | Did not lose target weight | 2 | LRYGBP | 32 (0.8) |
| 11 | F | 46 | 31 | Band erosion | 4 | LRYGBP | 29 (1.0) |
| 12 | F | 47 | 31 | Band erosion | 1.5 | LRYGBP | 30 (2.0) |
| 13 | F | 43 | 37 | Did not lose target weight | 4 | LRYGBP | 37 (0.1) |
| 14 | F | 53 | 39 | Band slippage/intolerance | 1.5 | LRYGBP | 35 (0.8) |
| 15 | F | 43 | 40 | Band slippage | 1.8 | LRYGBP | 36 (1.0) |
| 16 | M | 48 | 42 | Band erosion | 0.6 | LRYGBP | 34 (2.0) |
BMI = body mass index; F = female; M = male; NA = not applicable; LRYGBP = laparoscopic Roux-en-Y gastric bypass.
Discussion
As the epidemic of obesity continues to increase, it is important for bariatric surgery as a surgical discipline to establish robust outcomes for the different procedures available for weight control. The 2 most common procedures, LRYGBP and the LAGBD method, have been compared in this paper. Success after bariatric surgery is difficult to quantify. From a patient’s perspective, adequate and long-term sustainable weight loss and low mortality are essential factors. Our results show that both operations can be performed with acceptable mortality and low short-term complication rates. The type of surgery was not a significant variable contributing to increased mortality. This could be a false negative owing to the low number of events (death), the unequal sample size and the nonrandomized nature of the study, as this is a retrospective analysis of prospectively collected data. The overall complication rate in this study was higher in the LAGBD cohort, primarily owing to a statistically significant higher complication rate in the long term. Our findings are in keeping with those of Weber and colleagues27 (48% for LAGBD v. 15.7% for LRYGBP) and of Mognol and colleagues28 (26% for LAGBD v. 15.3% for LRYGBP). We had a higher occurrence of band erosions (4.0%) in this study compared with the literature (3%).29 We have no explanation for this other than technical factors in the early period of our learning curve. We have reviewed the operative recordings of all the band erosion cases and could identify 2 potential technical factors. One was damage to the gastric serosa in the area of the band from the holding/retraction graspers. The other could be the early technique of breaking off the very tip of the needle used for the gastro–gastric sutures in an attempt to reduce band leaks by inadvertent puncture of the band. The blunt tip of the needle required considerable force to puncture the stomach, which could cause sufficient damage to initiate a subclinical gastric leak and future erosion site. We abandoned this technique after the first 40 patients and have not seen band erosion in our last 100 patients.
Our rate of band explantations is not different from those reported in the literature.30,31 Some of the band complications such as the erosions, slips and leaks had to be treated with reoperation. The inclusion of band intolerance and/or inability to lose weight as a long-term complication is debatable. We feel that the availability of laparoscopic conversion of failed LAGBD to LRYGBP, unique to our program, accounts for the number of such conversions in our study. The bariatric team is less likely to diagnose or declare “band intolerance” if they have no capability to correct the problem with a back-up bariatric surgical procedure that can be performed by minimal invasive approaches. Our findings suggest that converting actual or perceived band failures to LRYGBP produces much better short-term results than revisions performed for perceived/actual failures (inability to lose the targeted weight) after gastric bypass. The revision of failed standard gastric bypass to distal gastric bypass did not produce the desired results. This is not surprising given our long-term follow-up of short versus long limb gastric bypass results.12
Weight loss greater than 50% of the excess weight32 or reduction of the BMI33 to less than 35 kg/m2 have been proposed as potential definitions of success of a bariatric surgical procedure. Our results suggest superiority in weight loss for LRYGBP versus the LAGBD method at all time intervals both as a mean of %EWL and by looking at individual groups of weight loss. At medium term (3 yr), 91% of the LRYGBP patients studied had achieved greater than 50% EWL compared with 62% in the LAGBD group. For the same period, mean %EWL for the LRYGBP group was 80% versus 59% for the LAGBD group. At every time point, the LRYGBP showed about 15% more %EWL than the LAGBD group. In assessing these results, it is important to take into consideration reoperation and reintervention rate. This was higher in the LAGBD group compared with the LRYGBP group, but the success rate of the revisional procedure from LAGBD to LRYGBP was much higher compared with the distal gastric bypass conversion of the RYGBP patients with insufficient weight loss. These results compare well to those reported by others.34–36 Another consideration is the rate of patient follow-up in our study. Despite our best efforts with the limited resources available, we were not able to follow up about one-third of our patients after 3 years. At least 40% of our patients come from a distance (> 4-h commute from Montréal). Our efforts to reach all our patients are ongoing, and we have gone to extremes of having the provincial health authority (all patients must be registered in order to have access to health care) or the local police authorities (using their internal databases) send letters to our patients on our behalf encouraging them to contact us. Since the follow-up rate is comparable in the 2 cohorts, we feel that the results reflect a real difference in weight loss outcomes as reported here.
It is important for future studies to identify robust predictors of successful weight loss for the procedure that will be offered to patients, thus avoiding disappointment, financial expenses, impairment in quality of life, and potential morbidity. Other series have reported on limited weight loss success with the LAGBD procedure and low quality of life.27,37–39 Our results are in keeping with pooled LAGBD series reporting 55% EWL at 5 years.21,40–42 Our LRYGBP weight loss data are in agreement with published large series of LRYGBP manifesting an identical 5-year 83% EWL.43,44 Our outcomes are similar to previous comparisons of the LAGBD procedure and LRYGBP in Europe41 and North America.45,46 They are also similar to the only prospective randomized trial of LAGBD versus LRYGBP reporting outcomes at 5 years postsurgery.47 This study comprised 51 patients, and all but 1 was followed up to 5 years. They found that, as in our study, the LRYGBP group had significantly better weight loss and a lower failure rate. Our 0.3% mortality is also within the reported 0.5% mortality rate as it has been verified from a large meta-analysis.48 We had no deaths in the LAGBD group. Though this procedure is promoted as “less complex” and “safer” than gastric bypass, mortality of LAGBD varies from 0.04%, as recently reported by Watkins and colleagues,49 up to 0.51%, as recently reported in a review by Gagner and colleagues.50 Some may argue that the LRYGBP is a more “radical” procedure, fraught with increased mortality. Indeed, one website in Canada quotes a mortality range of 3%–40% after gastric bypass,51 which is unsubstantiated. Mortalities after gastric bypass can be reduced by eliminating technical factors such as gastrointestinal leaks. After the last patient death from a leak (sequence no. 662), we instituted a new protocol of pneumatic testing of the pouch after formation under water, and methylene blue distention of the pouch and the gastrojejunostomy upon completion, followed by a final pneumatic test of both and the jejunojejunostomy under water, and we have not seen a postoperative leak in the remaining patients (> 400 overall to date).
Inability to achieve the weight loss goal after bariatric surgery is difficult to correct. The options are conversion to biliopancreatic diversion with duodenal switch,52 adjustable gastric band over bypass53 or distal gastric bypass (75–100 cm common channel). We have no experience with the first 2 revisions, as we converted all our LRYGBP patients who did not achieve adequate weight loss to laparoscopic distal gastric bypass with 100-cm common channel. Our results are not very encouraging and are in line with those reported by Brolin and Cody.54 We do not feel that our poor results are likely to improve with more follow-up. We feel there is something unique to the small numbers of gastric bypass patients who do not achieve at least 50% EWL that we as yet do not understand. Converting patients who did not lose weight after LAGBD surgery to LRYGBP produced acceptable reductions in weight and is in keeping with the findings reported by others.55–57 We have no experience with converting patients who were unsuccessfully treated with the LAGBD procedure to duodenal switch operations.52
Our study has certain limitations. It represents the personal series of 1 experienced bariatric surgeon’s minimally invasive laparoscopic bariatric surgery practice, including the learning curve.25 As such, there is no surgeon- or technique-related variability. It is not a randomized study, and as such it is subject to all the potential bias of a retrospective study. Despite our determined efforts to follow up on all our patients, we were not successful.Our study groups were of unequal size owing to personal preferences of the patients in selecting their surgical procedure. We also did not include in our analysis resolution and improvement of comorbidities, as our aim for this study was to concentrate on asessment of weight loss, and of morbidity and mortality. We intend to increase our efforts to complete the patient follow-up at the 10-year mark and include the analysis of the obesity-related comorbidity afflicting our patients. We have used this strategy successfully in the past.12,58
Conclusion
The results of this study demonstrate that both LRYGBP and the LAGBD method produce effective weight loss at 3 years. The LRYGBP method may produce better long-term weight loss if the 15% difference in weight loss identified here is maintained in the long term (5–10 yr). This method is also associated with lower overall and long-term complication rates. Given the limitations of our study, the better weight loss at 3–5 years of follow-up, as well as the lower overall and long-term complication and revision rates, suggests that LRYGBP may be the preferable procedure. The ideal bariatric procedure remains elusive as yet and requires further study.
Footnotes
Presented in part at the Canadian Surgical Forum in Halifax, NS, Sept. 10–13, 2008.
Competing intersts: Dr. Christou received an unrestricted educational grant from Ethicon Endosurgery Canada. Dr. Efthimiou’s fellowship was partially sponsored by Ethicon Endosurgery UK.
Contributors: Dr. Christou designed the study and acquired the data. Drs. Chrisou and Efthimiou both analyzed the data, wrote and reviewed the article, and approved its publication.
References
- 1.Rapport du groupe d’experts en santé publique, médecine et chirurgie bariatrique avec la collaboration des agences de la santé de la capitale nationale, de la Mauricie-Centre-Du-Québec et Montréal. L’organisation de la médecine et chirurgie bariatriques au Québec. 2007;1:1. [Google Scholar]
- 2.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i–xii):1–253. [PubMed] [Google Scholar]
- 3.Abelson P, Kennedy D. The obesity epidemic. Science. 2004;304:1413. doi: 10.1126/science.304.5676.1413. [DOI] [PubMed] [Google Scholar]
- 4.Torrance GM, Hooper MD, Reeder BA. Trends in overweight and obesity among adults in Canada (1970–1992) evidence from national surveys using measured height and weight. Int J Obes Relat Metab Disord. 2002;26:797–804. doi: 10.1038/sj.ijo.0801991. [DOI] [PubMed] [Google Scholar]
- 5.Katzmarzyk PT. The Canadian obesity epidemic, 1985–1998. CMAJ. 2002;166:1039–40. [PMC free article] [PubMed] [Google Scholar]
- 6.Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 7.Hazards of obesity. Nature. 1968;220:330. [PubMed] [Google Scholar]
- 8.Friedman JM. A war on obesity, not the obese. Science. 2003;299:856–8. doi: 10.1126/science.1079856. [DOI] [PubMed] [Google Scholar]
- 9.Fappa E, Yannakoulia M, Pitsavos C, et al. Lifestyle intervention in the management of metabolic syndrome: Could we improve adherence issues. Nutrition. 2008;24:286–91. doi: 10.1016/j.nut.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Hainer V, Toplak H, Mitrakou A. Treatment modalities of obesity: What fits whom? Diabetes Care. 2008;31(Suppl 2):S269–77. doi: 10.2337/dc08-s265. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Levine R, Troy L, et al. Three-year follow-up of participants in a commercial weight loss program. Can you keep it off. Arch Intern Med. 1996;156:1302–6. [PubMed] [Google Scholar]
- 12.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 14.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–23. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busetto L, Mirabelli D, Petroni ML, et al. Comparative long-term mortality after laparoscopic adjustable gastric banding versus nonsurgical controls. Surg Obes Relat Dis. 2007;3:496–502. doi: 10.1016/j.soard.2007.06.003. discussion 502. [DOI] [PubMed] [Google Scholar]
- 16.Peeters A, O’Brien PE, Laurie C, et al. Substantial intentional weight loss and mortality in the severely obese. Ann Surg. 2007;246:1028–33. doi: 10.1097/SLA.0b013e31814a6929. [DOI] [PubMed] [Google Scholar]
- 17.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 18.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 19.Sampalis JS, Liberman M, Auger S, et al. The impact of weight reduction surgery on health-care costs in morbidly obese patients. Obes Surg. 2004;14:939–47. doi: 10.1381/0960892041719662. [DOI] [PubMed] [Google Scholar]
- 20.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4:353–7. doi: 10.1381/096089294765558331. [DOI] [PubMed] [Google Scholar]
- 21.Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2008;4(Suppl):S47–55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Gastrointestinal surgery for severe obesity. [(accessed 2009 Oct. 7)];NIH Consens Statement Online. 1991 9:1–20. Available: http://consensus.nih.gov/1991/1991GISurgeryObesity084html.htm. [PubMed] [Google Scholar]
- 23.Deitel M, Greenstein RJ. Recommendations for reporting weight loss. Obes Surg. 2003;13:159–60. doi: 10.1381/096089203764467117. cas. [DOI] [PubMed] [Google Scholar]
- 24.metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;1983;64:3–9. [PubMed] [Google Scholar]
- 25.Andrew CG, Hanna W, Look D, et al. Early results after laparoscopic Roux-en-Y gastric bypass: effect of the learning curve. Can J Surg. 2006;49:417–21. [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Sabah S, Ladouceur M, Christou N. Anastomotic leaks after bariatric surgery: it is the host response that matters. Surg Obes Relat Dis. 2008;4:152–7. doi: 10.1016/j.soard.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Weber M, Muller MK, Bucher T, et al. Laparoscopic gastric bypass is superior to laparoscopic gastric banding for treatment of morbid obesity. Ann Surg. 2004;240:975–82. doi: 10.1097/01.sla.0000145924.64932.8f. discussion 982–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mognol P, Chosidow D, Marmuse JP. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: a comparative study of 290 patients. Obes Surg. 2005;15:76–81. doi: 10.1381/0960892052993486. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien PE, Dixon JB. Weight loss and early and late complications—the international experience. Am J Surg. 2002;184(6B):42S–5S. doi: 10.1016/s0002-9610(02)01179-0. [DOI] [PubMed] [Google Scholar]
- 30.Mognol P, Arapis KE. Long-term results after gastric banding: 12 years follow up. Surg Obes Relat Dis. 2008;4:292–3. [Google Scholar]
- 31.Dargent J. Isolated food intolerance after adjustable gastric banding: a major cause of long-term band removal. Obes Surg. 2008;18:829–32. doi: 10.1007/s11695-008-9495-x. [DOI] [PubMed] [Google Scholar]
- 32.Reinhold RB. Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155:385–94. [PubMed] [Google Scholar]
- 33.Biron S, Hould FS, Lebel S, et al. Twenty years of biliopancreatic diversion: What is the goal of the surgery. Obes Surg. 2004;14:160–4. doi: 10.1381/096089204322857492. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien P, Brown W, Dixon J. Revisional surgery for morbid obesity — conversion to the Lap-Band system. Obes Surg. 2000;10:557–63. doi: 10.1381/096089200321594174. [DOI] [PubMed] [Google Scholar]
- 35.Fobi MA, Lee H, Igwe D, Jr, et al. Revision of failed gastric bypass to distal Roux-en-Y gastric bypass: a review of 65 cases. Obes Surg. 2001;11:190–5. doi: 10.1381/096089201321577866. [DOI] [PubMed] [Google Scholar]
- 36.Slater GH, Fielding GA. Combining laparoscopic adjustable gastric banding and biliopancreatic diversion after failed bariatric surgery. Obes Surg. 2004;14:677–82. doi: 10.1381/096089204323093480. [DOI] [PubMed] [Google Scholar]
- 37.Biertho L, Steffen R, Branson R, et al. Management of failed adjustable gastric banding. Surgery. 2005;137:33–41. doi: 10.1016/j.surg.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Busetto L, Segato G, De Marchi F, et al. Outcome predictors in morbidly obese recipients of an adjustable gastric band. Obes Surg. 2002;12:83–92. doi: 10.1381/096089202321144649. [DOI] [PubMed] [Google Scholar]
- 39.Busetto L, Segato G, De Luca M, et al. Weight loss and postoperative complications in morbidly obese patients with binge eating disorder treated by laparoscopic adjustable gastric banding. Obes Surg. 2005;15:195–201. doi: 10.1381/0960892053268327. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–40. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 41.Biertho L, Steffen R, Ricklin T, et al. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding: a comparative study of 1,200 cases. J Am Coll Surg. 2003;197:536–44. doi: 10.1016/S1072-7515(03)00730-0. discussion 544–5. [DOI] [PubMed] [Google Scholar]
- 42.Cunneen SA, Phillips E, Fielding G, et al. Studies of Swedish adjustable gastric band and Lap-Band: systematic review and meta-analysis. Surg Obes Relat Dis. 2008;4:174–85. doi: 10.1016/j.soard.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y-500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–9. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–89. doi: 10.1097/00000658-200109000-00002. discussion 289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jan JC, Hong D, Bardaro SJ, et al. Comparative study between laparoscopic adjustable gastric banding and laparoscopic gastric bypass: single-institution, 5-year experience in bariatric surgery. Surg Obes Relat Dis. 2007;3:42–50. doi: 10.1016/j.soard.2006.11.005. discussion 50–1. [DOI] [PubMed] [Google Scholar]
- 46.Kim TH, Daud A, Ude AO, et al. Early U.S. outcomes of laparoscopic gastric bypass versus laparoscopic adjustable silicone gastric banding for morbid obesity. Surg Endosc. 2006;20:202–9. doi: 10.1007/s00464-005-0243-1. [DOI] [PubMed] [Google Scholar]
- 47.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3:127–32. doi: 10.1016/j.soard.2006.12.005. discussion 132–3. [DOI] [PubMed] [Google Scholar]
- 48.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 49.Watkins BM, Ahroni JH, Michaelson R, et al. Laparoscopic adjustable gastric banding in an ambulatory surgery center. Surg Obes Relat Dis. 2008;4(Suppl):S56–62. doi: 10.1016/j.soard.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Gagner M, Milone L, Yung E, et al. Causes of early mortality after laparoscopic adjustable gastric banding. J Am Coll Surg. 2008;206:664–9. doi: 10.1016/j.jamcollsurg.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 51.The LaparoscopicBAND Centre. [(accessed 2009 Oct. 7)]. [website of the TLBC]. Available: www.tlbc.ca/gastricband-banding.php.
- 52.Parikh M, Pomp A, Gagner M. Laparoscopic conversion of failed gastric bypass to duodenal switch: technical considerations and preliminary outcomes. Surg Obes Relat Dis. 2007;3:611–8. doi: 10.1016/j.soard.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Bessler M, Daud A, DiGiorgi MF, et al. Adjustable gastric banding as a revisional bariatric procedure after failed gastric bypass. Obes Surg. 2005;15:1443–8. doi: 10.1381/096089205774859173. [DOI] [PubMed] [Google Scholar]
- 54.Brolin RE, Cody RP. Weight loss outcome of revisional bariatric operations varies according to the primary procedure. Ann Surg. 2008;248:227–32. doi: 10.1097/SLA.0b013e3181820cdf. [DOI] [PubMed] [Google Scholar]
- 55.Gumbs AA, Pomp A, Gagner M. Revisional bariatric surgery for inadequate weight loss. Obes Surg. 2007;17:1137–45. doi: 10.1007/s11695-007-9209-9. [DOI] [PubMed] [Google Scholar]
- 56.Gagner M, Gumbs AA. Gastric banding: conversion to sleeve, bypass, or DS. Surg Endosc. 2007;21:1931–5. doi: 10.1007/s00464-007-9539-7. [DOI] [PubMed] [Google Scholar]
- 57.Gagner M, Gentileschi P, de Csepel J, et al. Laparoscopic reoperative bariatric surgery: experience from 27 consecutive patients. Obes Surg. 2002;12:254–60. doi: 10.1381/096089202762552737. [DOI] [PubMed] [Google Scholar]
- 58.MacLean LD, Rhode BM, Nohr CW. Long- or short-limb gastric bypass. J Gastrointest Surg. 2001;5:525–30. doi: 10.1016/s1091-255x(01)80091-3. [DOI] [PubMed] [Google Scholar]